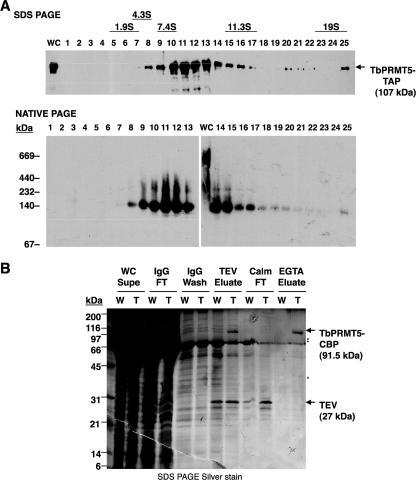
FIG. 7.
Analysis of in vivo TbPRMT5 protein complexes. (A) Glycerol gradient fractionation of TbPRMT5-TAP-containing complexes. Whole-cell extract prepared from PF trypanosomes ectopically expressing a TbPRMT5-TAP fusion protein was fractionated by centrifugation on a 5 to 20% glycerol gradient. The fractions were resolved by either denaturing polyacrylamide gel electrophoesis (SDS PAGE; top) or nondenaturing PAGE (native PAGE; bottom), and TbPRMT5-TAP-containing complexes were analyzed by immunoblotting using the TAP-specific PAP reagent. Twenty micrograms of whole-cell extract (WC) was analyzed by both methods for comparison. The sedimentation values and molecular masses of protein standards are indicated. (B) Tandem-affinity purification of native TbPRMT5 protein complexes. Wild type (W) or TbPRMT5-TAP (T) whole-cell extracts were fractionated by tandem-affinity purification over consecutive IgG-Sepharose and calmodulin resin columns. A silver-stained SDS-PAGE gel of TbPRMT5-CBP purification and complex composition is shown. TbPRMT5-CBP (arrow; 91.5 kDa) was eluted from IgG-Sepharose resin after TEV protease cleavage (TEV eluate), purified over calmodulin resin (Calm), and eluted under native conditions with EGTA (EGTA eluate). Major copurifying proteins present only in eluates of TAP-expressing cells (EGTA eluate; cf. lanes W and T) are indicated (asterisks). WC Supe, whole-cell supernatant; IgG FT, IgG-Sepharose resin flowthrough; TEV, TEV protease; Calm FT, calmodulin resin flowthrough.