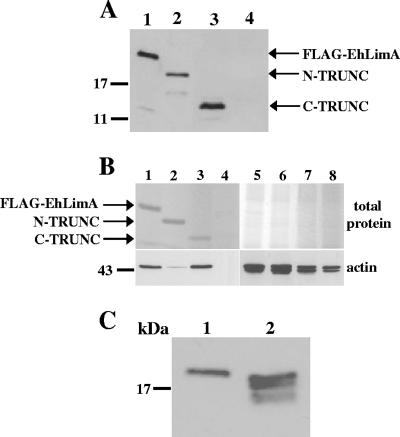
FIG. 7.
(A) Expression of N-terminus- and C-terminus-truncated FLAG-tagged EhLimA. Protein expression of N-terminus-truncated FLAG-tagged EhLimA (N-TRUNC, lane 2) and C-terminus-truncated FLAG-tagged EhLimA (C-TRUNC, lane 3) is confirmed in a Western blot analysis with anti-FLAG antibodies. In lane 1, expression of the full-length FLAG-EhLimA fusion protein is shown. Lane 4 contains a total cell lysate of wild-type cells. (B) Immunoprecipitation and actin binding of N-terminus- and C-terminus-truncated FLAG-tagged EhLimA. Total cell lysates of cells overexpressing FLAG- EhLimA (lane 1), N-TRUNC (lane 2), or C-TRUNC (lane 3) and wild-type cells (lane 4) were incubated with anti-FLAG monoclonal antibodies covalently attached to agarose. Immunoprecipitated proteins were analyzed by gel electrophoresis, followed by total protein staining of the gel (top gel). A Western blot analysis of immunoprecipitated protein products using antiactin antibodies reveals that N-TRUNC has almost completely lost the ability to bind actin (bottom gel). Protein amounts are confirmed in lanes 5 to 8 for lanes 1 to 4, respectively, each containing 10 μg of total lysate. (C) N-terminal cleavage of EhLimA. Anti-FLAG antibodies detect four bands in a Western blot of total cell lysates of cells overexpressing FLAG-tagged full-length EhLimA in which the FLAG epitope is located at the C terminus of the protein (C-FLAG, lane 2) as opposed to only one band that is detected in total cell lysates of the N-terminus-tagged FLAG-EhLimA-overexpressing cells (lane 1).