FIGURE 5. Preservation of the Cys110–Cys187 disulfide bond by nonspecific electrostatic effects.
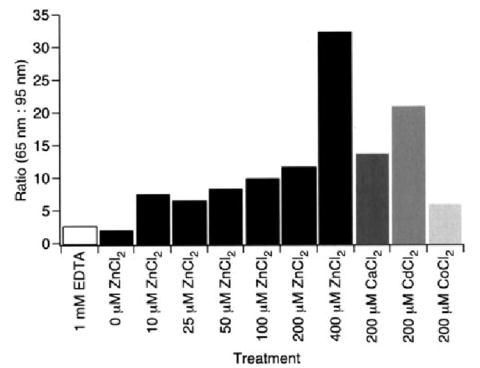
Ratios of F-D curves corresponding to the unfolding of rhodopsin in the presence (65 nm) and absence (95 nm) of the Cys110–Cys187 disulfide bond are shown. F-D curves were obtained from samples either in SMFS assay buffer supplemented with bivalent metal ions or in SMFS assay buffer without added MgCl2 and supplemented with 1 mm EDTA. The number of curves used to calculate the ratio is as follows: 1 mm EDTA, 264; 0 μm ZnCl2, 274; 10 μm ZnCl2, 187; 25 μm ZnCl2, 202; 50 μm ZnCl2, 222; 100 μm ZnCl2, 315; 200 μm ZnCl2, 114; 400 μm ZnCl2, 133; 200 μm CaCl2, 89; 200 μm CdCl2, 88; 200 μm CoCl2, 85.
