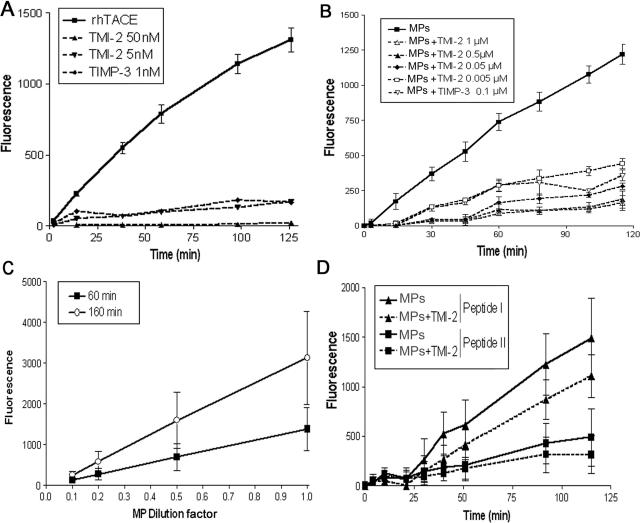
Figure 2.
MPs isolated from human atherosclerotic plaque are active on fluorogenic peptides that are substrates of TACE/ADAM17 or MMPs. Proteolytic activity of recombinant human TACE/ADAM17 and MPs were measured on various fluorogenic substrates. Details of assay conditions are indicated in Materials and Methods. A: Time-dependent cleavage by recombinant TACE/ADAM17 of the fluorogenic peptide III, mimetic of the cleavage zone of pro-TNF (10 ng) in the presence or not of TMI-2 at 5 and 50 nmol/L or TIMP-3 (100 nmol/L), mean ± SD of two separate measurements. B: Time-dependent cleavage by MPs (10 μl) of the fluorogenic peptide III in the presence of various concentrations of TMI-2, and TIMP-3. For the sake of clarity, data are presented only as the dose of 100 nmol/L TIMP-3 because 200 nmol/L gave similar inhibition, and SEM in place of SD to avoid overlapping of error bars. n = 4. C: Dose-dependent effect of MPs [expressed as fold of the maximal amount used (10 μl)] on the cleavage of the fluorogenic peptide III. For clarity of the graph, only two time points are presented. Values are mean ± SD of two separate MP preparations. D: Time-dependent cleavage by MPs of fluorogenic peptide I and peptide II in the presence or not of TMI-2 (1 μmol/L). Values are mean ± SD of four separate MP preparations identical to those used in B.