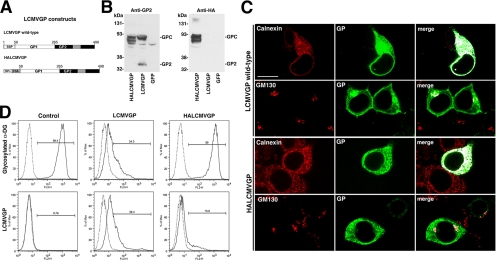
Figure 6.
Maturation of the viral GP is required for its ability to interfere with the expression of functional α-DG. (A) Schematic representation of wild-type LCMVGP and HALCMVGP. The transmembrane domain (gray box), the SSP, the Igκ signal peptide (SPκ), and the HA epitope are indicated. (B) Expression of wild-type LCMVGP and HALCMVGP: HEK293T cells were transfected with wild-type LCMVGP, HALCMVGP, or GFP. Total cell protein was extracted after 48 h and analyzed in Western blot by using mAb 83.6 (Anti-GP2) and mAb F7 to HA epitope (anti-HA). Molecular masses and the positions of the GPC and mature GP2 are indicated. (C) Cellular localization of wild-type LCMVGP and HALCMVGP: HEK293T cells were transfected with wild-type LCMVGP and HALCMVGP. After 48 h, cells were fixed, permeabilized, and stained for LCMVGP with mAb 83.6 (mouse IgG2a) to LCMV GP2 combined with mAbs to the ER marker calnexin (mouse IgG1) and the Golgi marker GM130 (mouse IgG1). Primary antibodies were detected with IgG subclass-specific secondary antibodies conjugated to FITC (green) and Rhodamine Red-X (red). Images were analyzed using LSM Image Examiner and ImageJ. Overlap of red and green signals are white in the merged image. Bar, 10 μm. (D) Detection of LCMVGP and glycosylated α-DG at the cell surface: HEK293T cells were transfected with empty vector (mock), LCMVGP, and HALCMVGP. After 48 h, cells were subjected to cell surface staining with mAb 83.6 (anti-GP) and mAb IIH6 (glycosylated α-DG) as in described in Figure 2D.