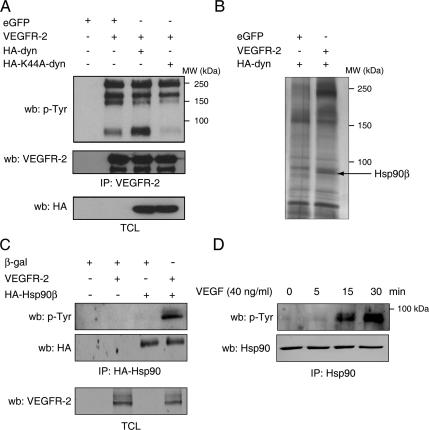
Figure 3.
VEGF induces tyrosine phosphorylation of Hsp90β. (A) COS-7 cells were cotransfected with VEGFR-2, eGFP, as a control, and HA-dynamin (HA-dyn) and HA-K44A-dynamin (HA-K44A-dyn) expression vectors as indicated. Cell lysates were immunoprecipitated (IP) with an anti-VEGFR-2 antibody and Western blotted (wb) using anti-phosphotyrosine antibodies (p-Tyr). Equal protein expression was determined by Western blotting the total cell lysates (TCL). (B) VEGFR-2 internalization is necessary for Hsp90 phosphorylation. VEGFR-2 immunoprecipitate from COS-7 cells, cotransfected as in A, were separated by SDS-PAGE, and proteins were detected by silver staining. The unique ≈85-kDa protein present in the VEGFR-2–transfected cells (arrow) was cut, subjected to LC/MS/MS analysis, and identified as Hsp90β. (C) VEGFR-2 stimulates tyrosine phosphorylation of Hsp90β. HA-Hsp90β immunoprecipitation was done in COS-7 cell lysates using and levels of tyrosine phosphorylation detected by anti-p-Tyr Western blotting. (D) VEGF stimulation of endothelial cells induces tyrosine phosphorylation of Hsp90. BAECs were stimulated with VEGF (40 ng/ml) for the indicated time and Hsp90 was immunoprecipitated from the cell extracts and Western blotted using anti-p-Tyr.