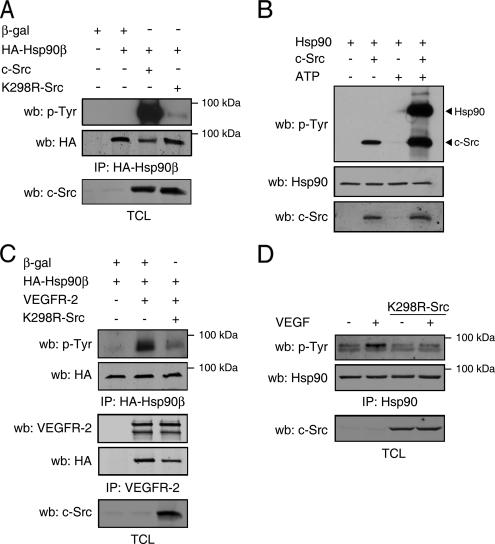
Figure 5.
c-Src mediates the VEGF-stimulated tyrosine phosphorylation of Hsp90. (A) c-Src expression induces tyrosine phosphorylation of Hsp90β. COS-7 cells were transfected as indicated and cell extracts were immunoprecipitated (IP) using anti-HA. HA-Hsp90β tyrosine phosphorylation was determined by Western blotting using anti-p-Tyr. (B) c-Src phosphorylates Hsp90 in vitro. Purified Hsp90 was incubated with recombinant c-Src in the presence or absence of ATP. The kinase reaction was then immunoblotted with anti-p-Tyr, anti-Hsp90, or anti-c-Src antibodies. (C) Src activity is necessary for VEGFR-2–stimulated phosphorylation of Hsp90β. COS-7 cells were transfected with HA-Hsp90β, VEGFR-2, and K298R-Src. Lysates were immunoprecipitated with anti-HA or anti-VEGFR-2 antibodies and immunoblotted with anti-p-Tyr or anti-HA. (D) c-Src activity is necessary for VEGF-stimulated phosphorylation of Hsp90 in endothelial cells. BAECs were transiently transfected with K298R-Src–expressing vector. Serum-starved cells were stimulated with VEGF (40 ng/ml, 15 min,) and cell lysates were immunoprecipitated using anti-Hsp90 antibody and immunoblotted using anti-p-Tyr.