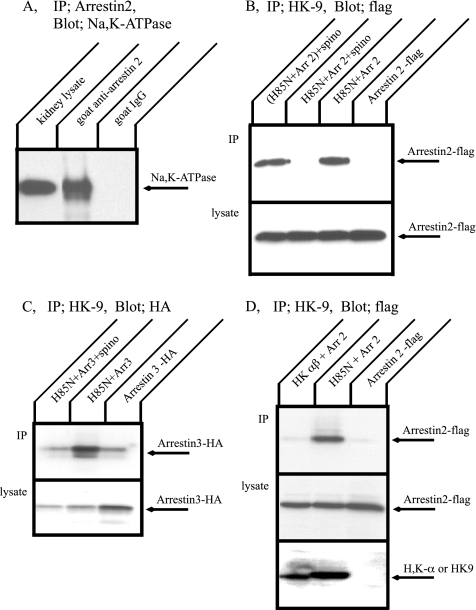
Figure 4.
Immunoprecipitation of the Na+,K+-ATPase and arrestin. (A) Mouse kidney lysate was incubated with a goat antibody directed against arrestin 2 or with nonimmune goat serum (control) followed by protein A beads. Immune complexes were separated by SDS-PAGE, and Western blot was performed with anti Na+,K+-ATPase antibody, α5. The Na+,K+-ATPase was coprecipitated with arrestin 2. (B) COS cells were transfected with flag-arrestin 2 alone, H85N plus flag-arrestin 2, or H85N, flag-arrestin 2, and spinophilin. Immunoprecipitation was performed with HK9 antibody directed against the N terminus of H85N, and arrestin 2 was detected by Western blotting with anti-flag antibody. Arrestin 2 was pulled down with the Na+,K+-ATPase α-subunit. This association, however, was inhibited by coexpression of spinophilin. (C) COS cells were transfected with HA-arrestin 3 alone, H85N plus HA-arrestin 3, or H85N, HA-arrestin 3 and spinophilin. Immunoprecipitation was performed with HK9 antibody and arrestin 3 was detected by Western blotting with anti-HA antibody. Arrestin 3 was also coimmunoprecipitated with the Na+,K+-ATPase, and this association was blocked by coexpression of spinophilin. (D) COS cells were transfected with flag-arrestin 2 alone or H+,K+-ATPase α- and β-subunits plus flag-arrestin 2. Immunoprecipitation was performed with HK9 antibody and arrestin 2 was detected by Western blotting with anti-flag antibody. Arrestin 2 was not immunoprecipitated with the H+,K+-ATPase. Typical results from one of five experiments are shown.