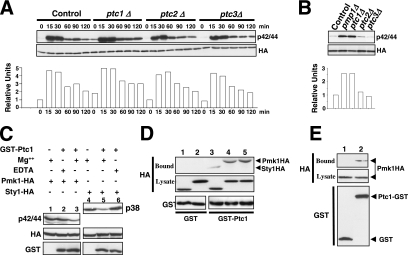
Figure 4.
Ptc1p associates with, and dephosphorylates, Pmk1p. (A) Strains MI200 (pmk1-HA6H, Control), MI216 (pmk1-HA6H, ptc1Δ), MI218 (pmk1-HA6H, ptc2Δ), and MI219 (pmk1-HA6H, ptc3Δ) were grown in YES medium to mid-log phase and treated with 0.6 M KCl. At different times Pmk1-HA6H was purified by affinity chromatography, and either activated or total Pmk1p was detected by immunoblotting with anti-phospho-p42/44 or anti-HA antibodies, respectively. (B) Strains MI200 (pmk1-HA6H, Control), MI212 (pmk1-HA6H, pmp1Δ), MI216 (pmk1-HA6H, ptc1Δ), MI218 (pmk1-HA6H, ptc2Δ), and MI219 (pmk1-HA6H, ptc3Δ) were grown in YES medium to mid-log phase, and Pmk1p basal activity was determined by using anti-phospho-p42/44 antibody as described above. (C) Pmk1p dephosphorylation in vitro. Activated Pmk1-HA6H or Sty1-HA6H was purified with Ni2+-NTA-agarose beads from strains MI200 (Pmk1p; lanes 1–3) or JM1521 (Sty1p; lanes 4–6) after treatment with 0.6 M KCl for 15 min. The beads were incubated at 30°C for 45 min in phosphatase buffer with 10 μg of GST-Ptc1 (lanes 2, 3, 5, and 6) in the presence of 10 mM MgCl2 (lanes 3 and 5) or 10 mM EDTA (lanes 2 and 6). The samples were analyzed by SDS-PAGE and immunoblotted with anti-p42/44, anti-HA, and anti-GST antibodies. (D) Pmk1p and Ptc1p interact in vitro. Cell lysates from exponentially growing strains JM1521 (Sty1-HA6H; lanes 1 and 3), MI200 (Pmk1-HA6H; lanes 2 and 4), and strain MI200 subjected to a 15-min treatment with 0.6 M KCl (lane 5) were incubated each with 15 μg of bacterially purified GST (lanes 1 and 2) or GST-Ptc1 (lanes 3–5) bound to glutathione-Sepharose beads. The beads were washed extensively, and the binding of Pmk1p or Sty1p fusions was detected by inmunoblotting with monoclonal anti-HA antibodies. (E) Pmk1p and Ptc1p interact in vivo. An MI200 transformant expressing unfused GST from plasmid pDS472a (lane 1) and strain MI505 (pmk1-HA6H, ptc1-GST) expressing a genomic version of Ptc1p fused to GST at its C terminus (lane 2) were grown in EMM2 medium in the absence of thiamine for 16 h. GST and Pyp1-GST fusions were purified with glutathione-Sepharose beads, resolved by SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with anti-HA or anti-GST antibodies.