Abstract
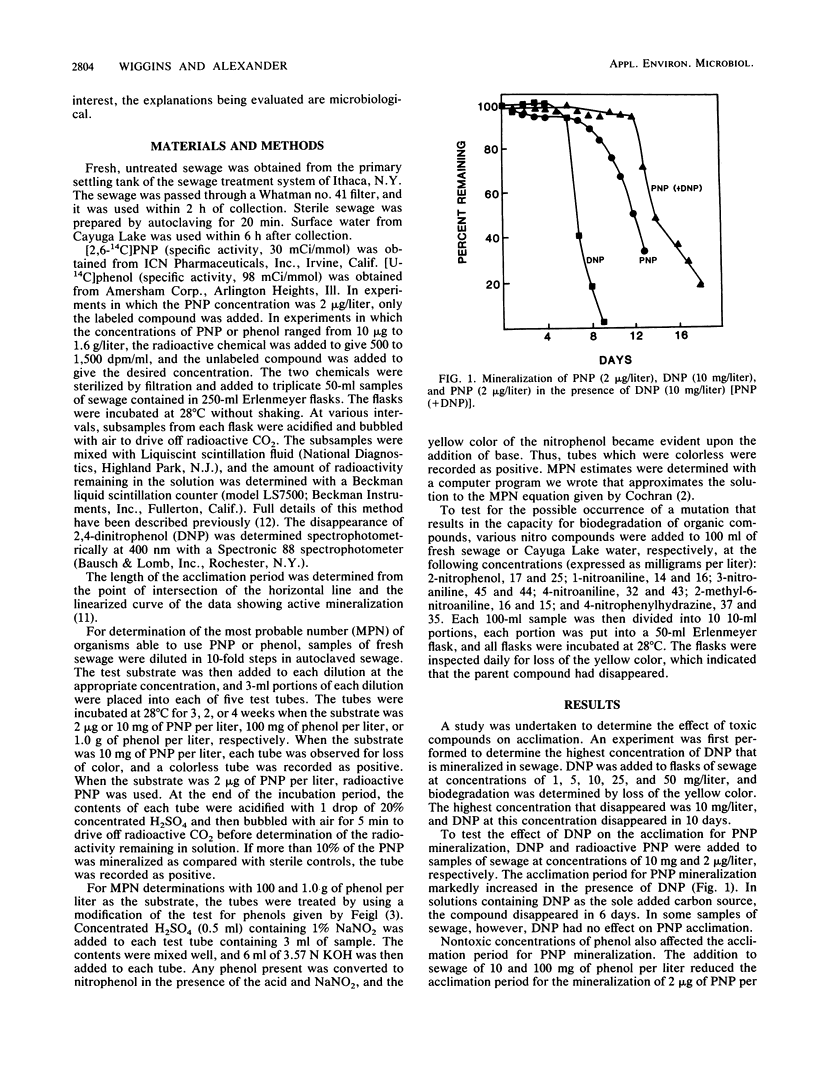
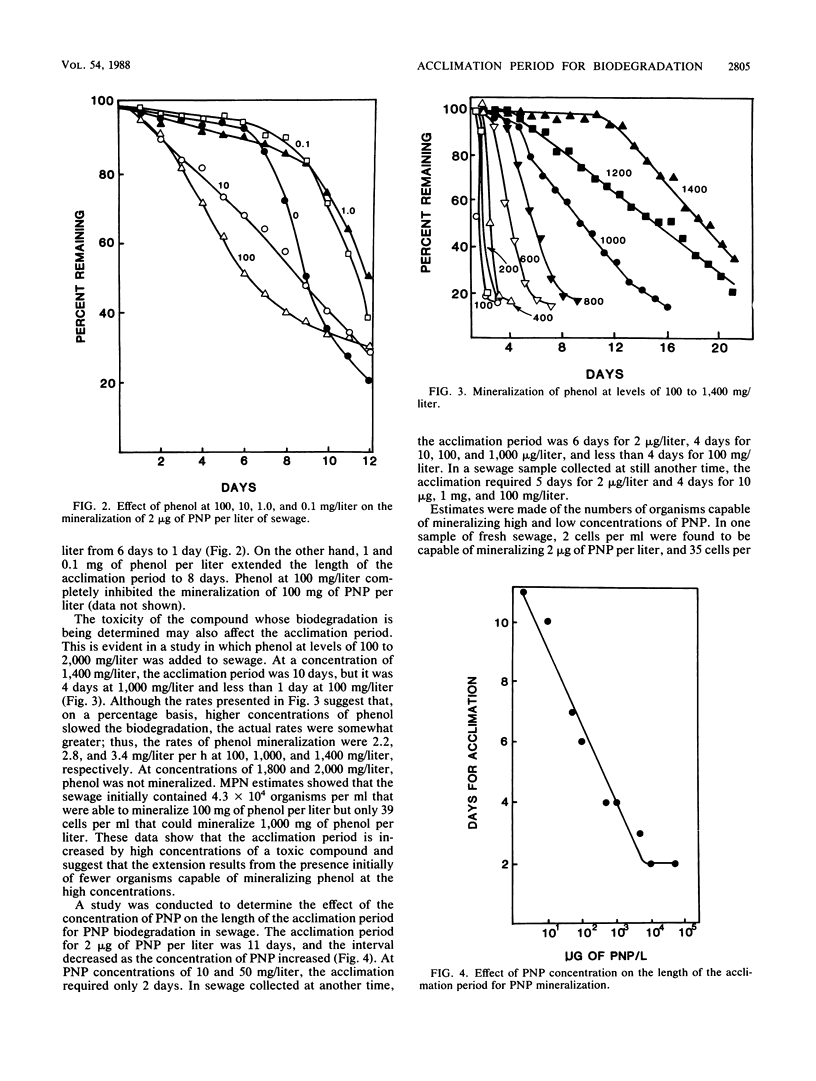
A study was conducted to determine the role of concentration of the test chemical, of a second organic compound, and of mutation in the acclimation period before the mineralization of organic compounds in sewage. The acclimation period for the mineralization in sewage of 2 micrograms of 4-nitrophenol (PNP) per liter increased from 6 to 12 days in the presence of 10 mg of 2,4-dinitrophenol per liter. The extension of the acclimation period was equivalent to the time required for mineralization of 2,4-dinitrophenol. In contrast, the time for acclimation for the degradation of 2 micrograms of PNP per liter was reduced when 10 or 100 mg of phenol per liter was added. Lower phenol levels increased the acclimation period to 8 days. The length of the acclimation period for PNP mineralization decreased as the initial concentration of PNP increased from 2 micrograms to 100 mg/liter. The acclimation period for phenol mineralization was lengthened as the phenol concentration increased from 100 to 1,400 mg/liter. The length of the acclimation period for PNP and phenol biodegradation was reproducible, but it varied among replicates for the biodegradation of other nitro-substituted compounds added to sewage or lake water, suggesting that a mutation was responsible for acclimation to these other compounds. The acclimation period may thus reflect the time required for the destruction of toxins, and it also may be affected by the concentration of the test compound or the presence of other substrates.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atlas R. M., Bartha R. Biodegradation of petroleum in seawater at low temperatures. Can J Microbiol. 1972 Dec;18(12):1851–1855. doi: 10.1139/m72-289. [DOI] [PubMed] [Google Scholar]
- COCHRAN W. G. Estimation of bacterial densities by means of the "most probable number". Biometrics. 1950 Jun;6(2):105–116. [PubMed] [Google Scholar]
- Lappin H. M., Greaves M. P., Slater J. H. Degradation of the herbicide mecoprop [2-(2-methyl-4-chlorophenoxy)propionic Acid] by a synergistic microbial community. Appl Environ Microbiol. 1985 Feb;49(2):429–433. doi: 10.1128/aem.49.2.429-433.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S. K., Simkins S., Alexander M. Models for the kinetics of biodegradation of organic compounds not supporting growth. Appl Environ Microbiol. 1985 Aug;50(2):323–331. doi: 10.1128/aem.50.2.323-331.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkins S., Alexander M. Models for mineralization kinetics with the variables of substrate concentration and population density. Appl Environ Microbiol. 1984 Jun;47(6):1299–1306. doi: 10.1128/aem.47.6.1299-1306.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain J. C., Van Veld P. A., Monti C. A., Pritchard P. H., Cripe C. R. Comparison of p-Nitrophenol Biodegradation in Field and Laboratory Test Systems. Appl Environ Microbiol. 1984 Nov;48(5):944–950. doi: 10.1128/aem.48.5.944-950.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subba-Rao R. V., Rubin H. E., Alexander M. Kinetics and extent of mineralization of organic chemicals at trace levels in freshwater and sewage. Appl Environ Microbiol. 1982 May;43(5):1139–1150. doi: 10.1128/aem.43.5.1139-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins B. A., Jones S. H., Alexander M. Explanations for the acclimation period preceding the mineralization of organic chemicals in aquatic environments. Appl Environ Microbiol. 1987 Apr;53(4):791–796. doi: 10.1128/aem.53.4.791-796.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyndham R. C. Evolved aniline catabolism in Acinetobacter calcoaceticus during continuous culture of river water. Appl Environ Microbiol. 1986 Apr;51(4):781–789. doi: 10.1128/aem.51.4.781-789.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


