Abstract
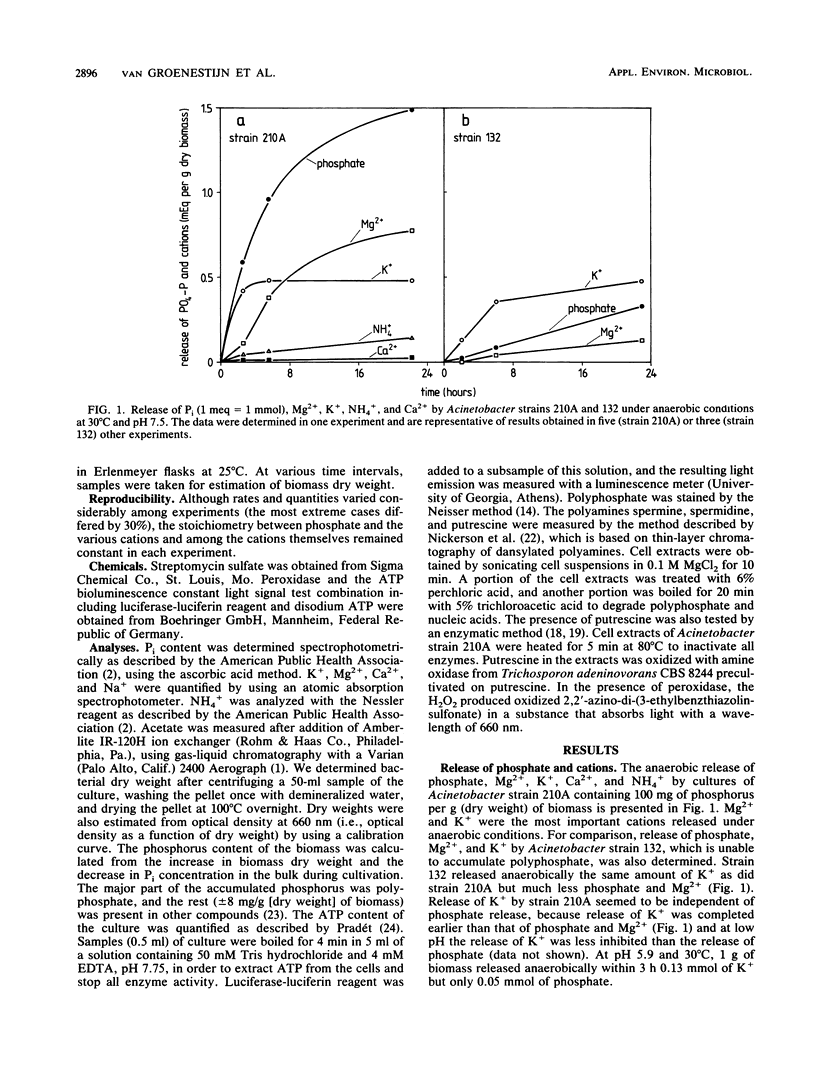
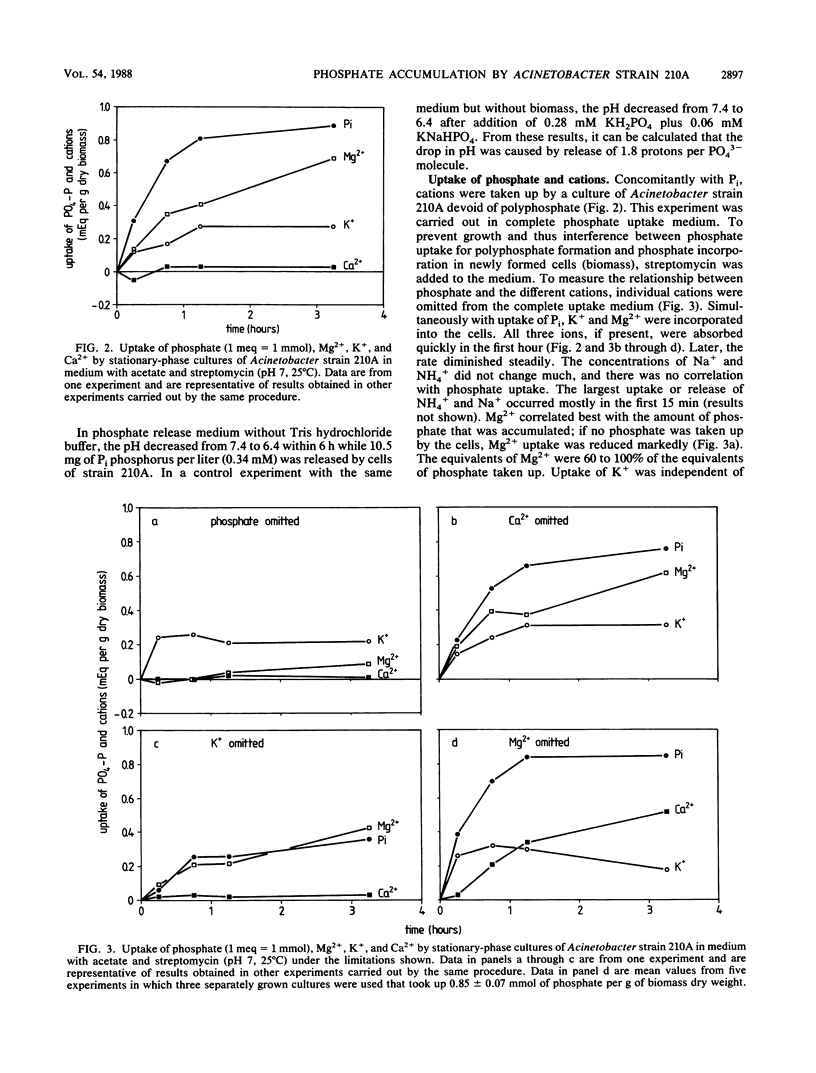
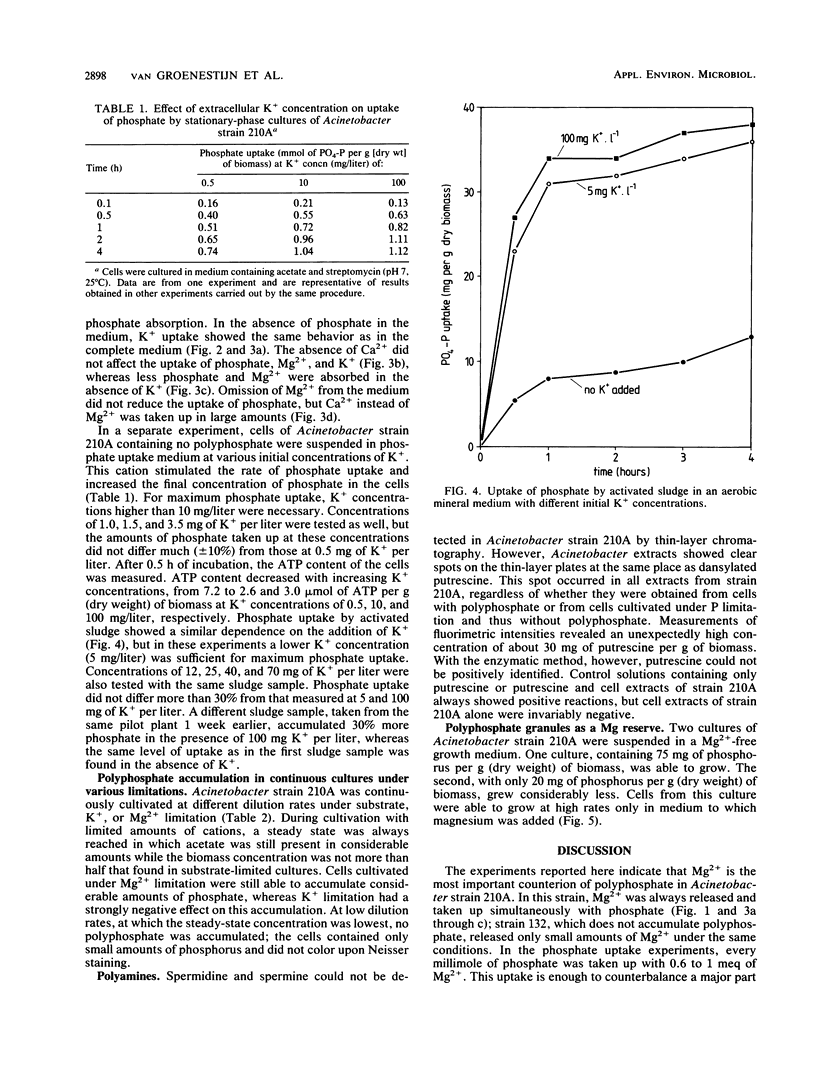
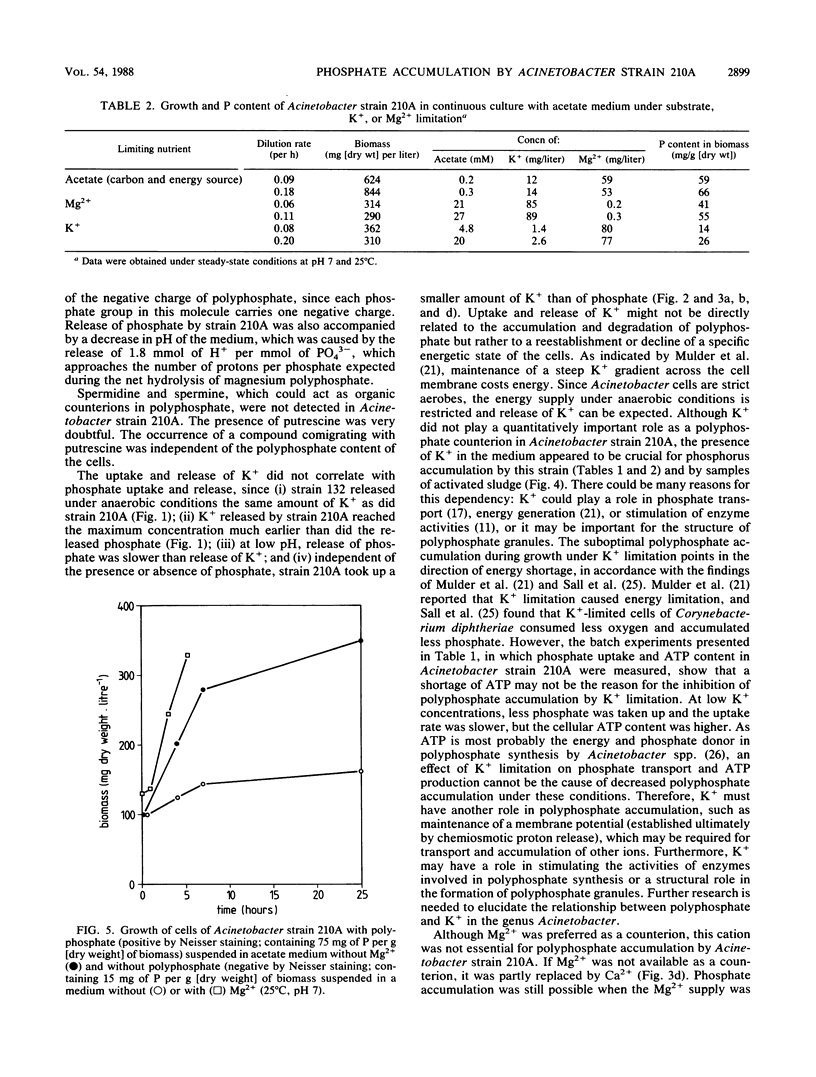
Cells of the strictly aerobic Acinetobacter strain 210A, containing aerobically large amounts of polyphosphate (100 mg of phosphorus per g [dry weight] of biomass), released in the absence of oxygen 1.49 mmol of Pi, 0.77 meq of Mg2+, 0.48 meq of K+, 0.02 meq of Ca2+, and 0.14 meq of NH4+ per g (dry weight) of biomass. The drop in pH during this anaerobic phase was caused by the release of 1.8 protons per PO43− molecule. Cells of Acinetobacter strain 132, which do not accumulate polyphosphate aerobically, released only 0.33 mmol of Pi and 0.13 meq of Mg2+ per g (dry weight) of biomass but released K+ in amounts comparable to those released by strain 210A. Stationary-phase cultures of Acinetobacter strain 210A, in which polyphosphate could not be detected by Neisser staining, aerobically took up phosphate simultaneously with Mg2+, the most important counterion in polyphosphate. In the absence of dissolved phosphate in the medium, no Mg2+ was taken up. Cells containing polyphosphate granules were able to grow in a Mg-free medium, whereas cells without these granules were not. Mg2+ was not essential as a counterion because it could be replaced by Ca2+. The presence of small amounts of K+ was essential for polyphosphate formation in cells of strain 210A. During continuous cultivation under K+ limitation, cells of Acinetobacter strain 210A contained only 14 mg of phosphorus per g (dry weight) of biomass, whereas this element was accumulated in amounts of 59 mg/g under substrate limitation and 41 mg/g under Mg2+ limitation. For phosphate uptake in activated sludge, the presence of K+ seemed to be crucial.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamse A. D. New isolation of Clostridium aceticum (Wieringa). Antonie Van Leeuwenhoek. 1980;46(6):523–531. doi: 10.1007/BF00394009. [DOI] [PubMed] [Google Scholar]
- Cembella A. D., Antia N. J., Harrison P. J. The utilization of inorganic and organic phosphorus compounds as nutrients by eukaryotic microalgae: a multidisciplinary perspective. Part 2. Crit Rev Microbiol. 1984;11(1):13–81. doi: 10.3109/10408418409105902. [DOI] [PubMed] [Google Scholar]
- Cramer C. L., Davis R. H. Polyphosphate-cation interaction in the amino acid-containing vacuole of Neurospora crassa. J Biol Chem. 1984 Apr 25;259(8):5152–5157. [PubMed] [Google Scholar]
- Epstein W., Davies M. Potassium-dependant mutants of Escherichia coli K-12. J Bacteriol. 1970 Mar;101(3):836–843. doi: 10.1128/jb.101.3.836-843.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg I., Avigad G. Structures containing polyphosphate in Micrococcus lysodeikticus. J Bacteriol. 1968 Aug;96(2):544–553. doi: 10.1128/jb.96.2.544-553.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. E., Chambers L. A. Localized intracellular polyphosphate formation by Desulfovibrio gigas. J Gen Microbiol. 1975 Jul;89(1):67–72. doi: 10.1099/00221287-89-1-67. [DOI] [PubMed] [Google Scholar]
- Medveczky N., Rosenberg H. Phosphate transport in Escherichia coli. Biochim Biophys Acta. 1971 Aug 13;241(2):494–506. doi: 10.1016/0005-2736(71)90048-4. [DOI] [PubMed] [Google Scholar]
- Middlehoven W. J., Hoogkamer-Te Niet M. C., De Laat W. T., Weijers C., Bulder C. J. Oxidation of amines by yeasts grown on 1-aminoalkanes or putrescine as the sole source of carbon, nitrogen and energy. Antonie Van Leeuwenhoek. 1986;52(6):525–535. doi: 10.1007/BF00423413. [DOI] [PubMed] [Google Scholar]
- Mulder M. M., Teixeira de Mattos M. J., Postma P. W., van Dam K. Energetic consequences of multiple K+ uptake systems in Escherichia coli. Biochim Biophys Acta. 1986 Sep 10;851(2):223–228. doi: 10.1016/0005-2728(86)90129-5. [DOI] [PubMed] [Google Scholar]
- Nickerson K. W., Dunkle L. D., Van Etten J. L. Absence of spermine in filamentous fungi. J Bacteriol. 1977 Jan;129(1):173–176. doi: 10.1128/jb.129.1.173-176.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALL T., MUDD S., DAVIS J. C. Factors conditioning the accumulation and disappearance of metaphosphate in cells of Corynebacterium diphtheriae. Arch Biochem Biophys. 1956 Jan;60(1):130–146. doi: 10.1016/0003-9861(56)90405-2. [DOI] [PubMed] [Google Scholar]


