Abstract
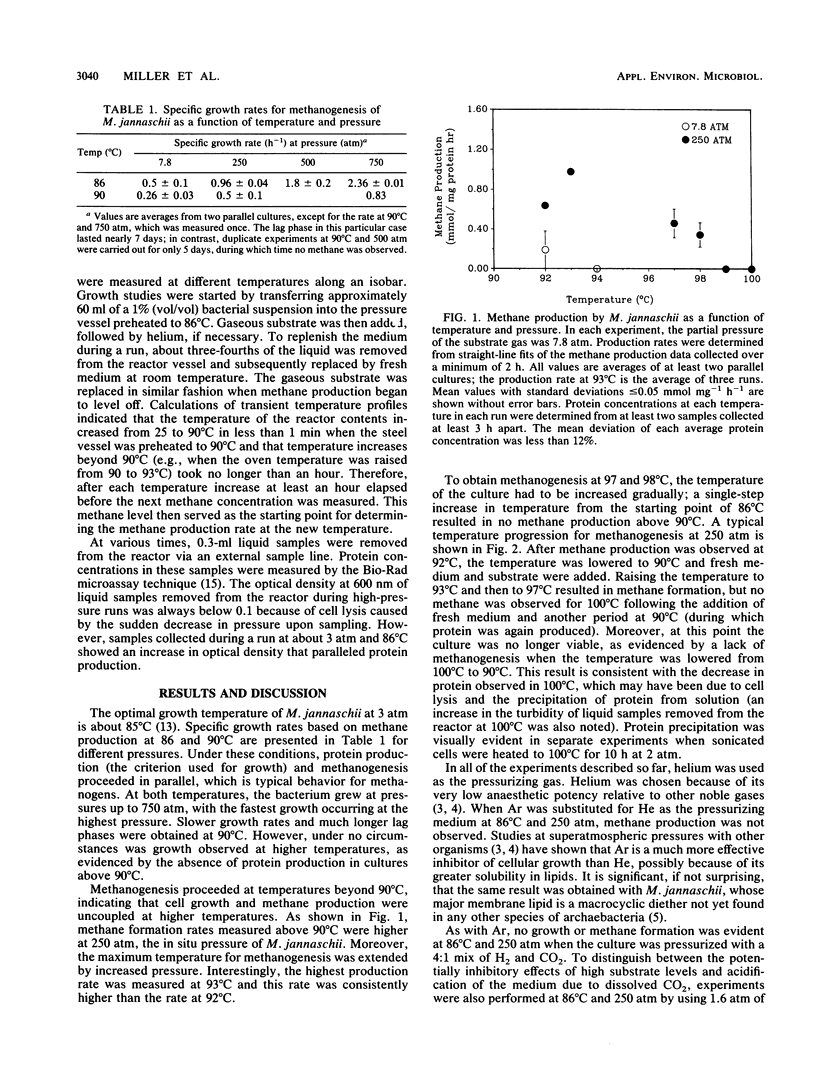
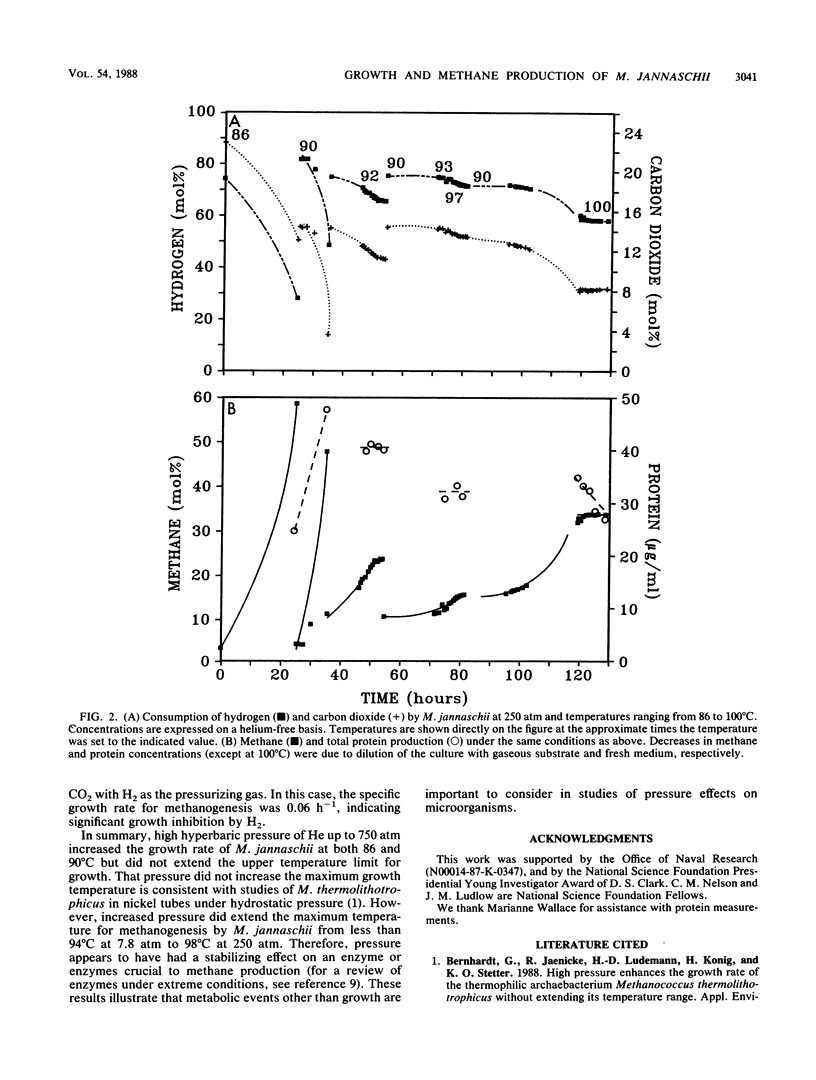
The marine archaebacterium Methanococcus jannaschii was studied at high temperatures and hyperbaric pressures of helium to investigate the effect of pressure on the behavior of a deep-sea thermophile. Methanogenesis and growth (as measured by protein production) at both 86 and 90°C were accelerated by pressure up to 750 atm (1 atm = 101.29kPa), but growth was not observed above 90°C at either 7.8 or 250 atm. However, growth and methanogenesis were uncoupled above 90°C, and the high-temperature limit for methanogenesis was increased by pressure. Substantial methane formation was evident at 98°C and 250 atm, whereas no methane formation was observed at 94°C and 7.8 atm. In contrast, when argon was substituted for helium as the pressurizing gas at 250 atm, no methane was produced at 86°C. Methanogenesis was also suppressed at 86°C and 250 atm when the culture was pressurized with a 4:1 mix of H2 and CO2, although limited methanogenesis did occur when the culture was pressurized with H2.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brock T. D. Life at high temperatures. Science. 1985 Oct 11;230(4722):132–138. doi: 10.1126/science.230.4722.132. [DOI] [PubMed] [Google Scholar]
- Bruemmer J. H., Brunetti B. B., Schreiner H. R. Effects of helium group gases and nitrous oxide on HeLa cells. J Cell Physiol. 1967 Jun;69(3):385–392. doi: 10.1002/jcp.1040690315. [DOI] [PubMed] [Google Scholar]
- Buchheit R. G., Schreiner H. R., Doebbler G. F. Growth responses of Neurospora crassa to increased partial pressures of the noble gases and nitrogen. J Bacteriol. 1966 Feb;91(2):622–627. doi: 10.1128/jb.91.2.622-627.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comita P. B., Gagosian R. B. Membrane lipid from deep-sea hydrothermal vent methanogen: a new macrocyclic glycerol diether. Science. 1983 Dec 23;222(4630):1329–1331. doi: 10.1126/science.222.4630.1329. [DOI] [PubMed] [Google Scholar]
- Deming J. W., Baross J. A. Solid Medium for Culturing Black Smoker Bacteria at Temperatures to 120 degrees C. Appl Environ Microbiol. 1986 Feb;51(2):238–243. doi: 10.1128/aem.51.2.238-243.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenicke R. Enzymes under extremes of physical conditions. Annu Rev Biophys Bioeng. 1981;10:1–67. doi: 10.1146/annurev.bb.10.060181.000245. [DOI] [PubMed] [Google Scholar]
- Jannasch H. W., Mottl M. J. Geomicrobiology of deep-sea hydrothermal vents. Science. 1985 Aug 23;229(4715):717–725. doi: 10.1126/science.229.4715.717. [DOI] [PubMed] [Google Scholar]
- Jannasch H. W., Wirsen C. O., Molyneaux S. J., Langworthy T. A. Extremely thermophilic fermentative archaebacteria of the genus desulfurococcus from deep-sea hydrothermal vents. Appl Environ Microbiol. 1988 May;54(5):1203–1209. doi: 10.1128/aem.54.5.1203-1209.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayanos A. A. Evolutional and ecological implications of the properties of deep-sea barophilic bacteria. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9542–9546. doi: 10.1073/pnas.83.24.9542. [DOI] [PMC free article] [PubMed] [Google Scholar]


