Abstract
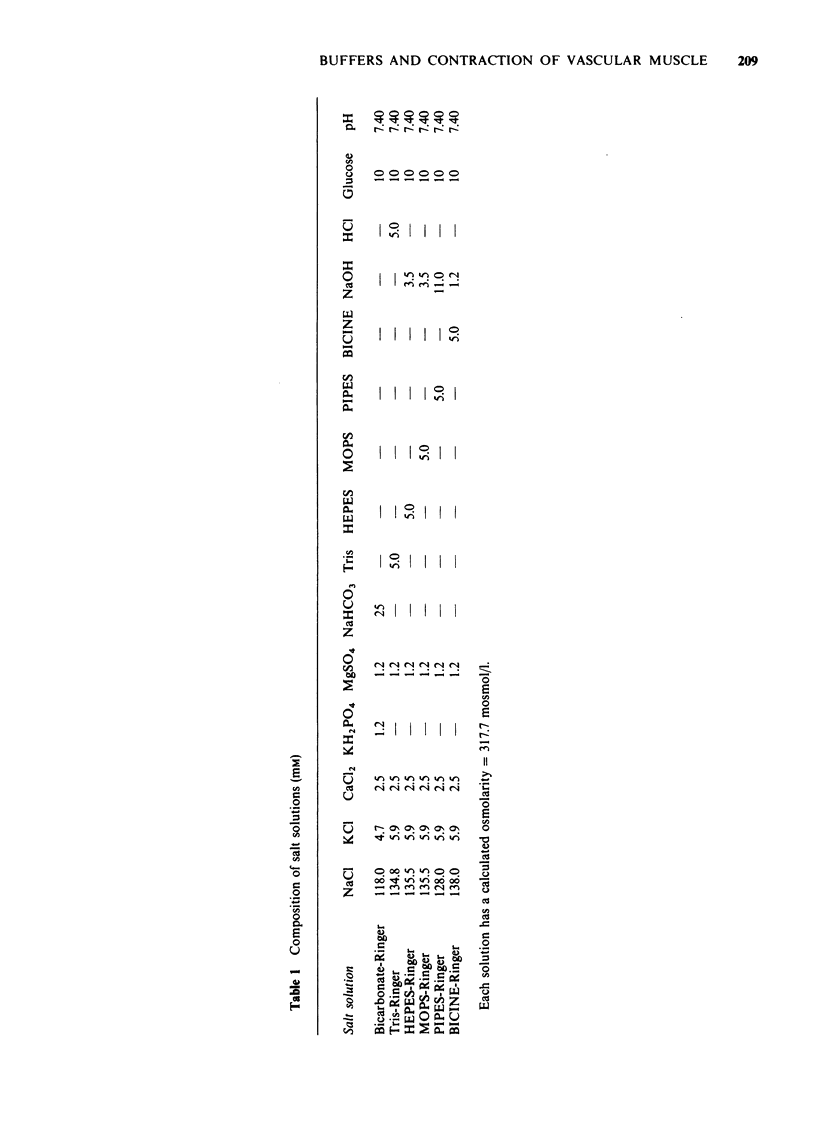
1 In vitro studies were undertaken on rat aortic strips and portal vein segments in order to determine whether or not several commonly used artificial buffers, i.e., tris(hydroxymethyl) aminomethane (Tris), N-2-hydroxyethylpiperazine-N'-2-ethanesulphonic acid (HEPES), morpholine propanesulphonic acid (MOPS), N,N bis(2-hydroxyethyl) glycine (BICINE) and 1,4-piperazinediethanesulphonic acid (PIPES), can exert direct actions on vascular smooth muscle. 2 All artificial buffers used in 5 mM concentrations were found to inhibit development of spontaneous mechanical activity. 3 Tris, HEPES, MOPS, BICINE and PIPES markedly attenuated contractions induced by adrenaline, angiotensin and KCl. The fast phase components of the agonist-induced contractions were either obliterated or reduced in the presence of the artificial buffers. The sustained slow phase components were greatly reduced and retarded by the artificial buffers. 4 The relative order of artificial buffer potency (i.e., from 100% to 14% inhibition) seems to depend upon the agonist and type of smooth muscle. 5 All of these inhibitory effects were reversible, since normal contractile responses and spontaneous mechanical activity could be obtained by simply reincubating the smooth muscles in Krebs-Ringer bicarbonate buffer. 6 A variety of pharmacological antagonists failed to mimic or affect the inhibitory effects of Tris, HEPES, MOPS, PIPES and BICINE. 7 These data show that five of the most commonly used artificial buffers, to study muscles in vitro, exert adverse effects on contractility of arterial and venous smooth muscle.
Full text
PDF


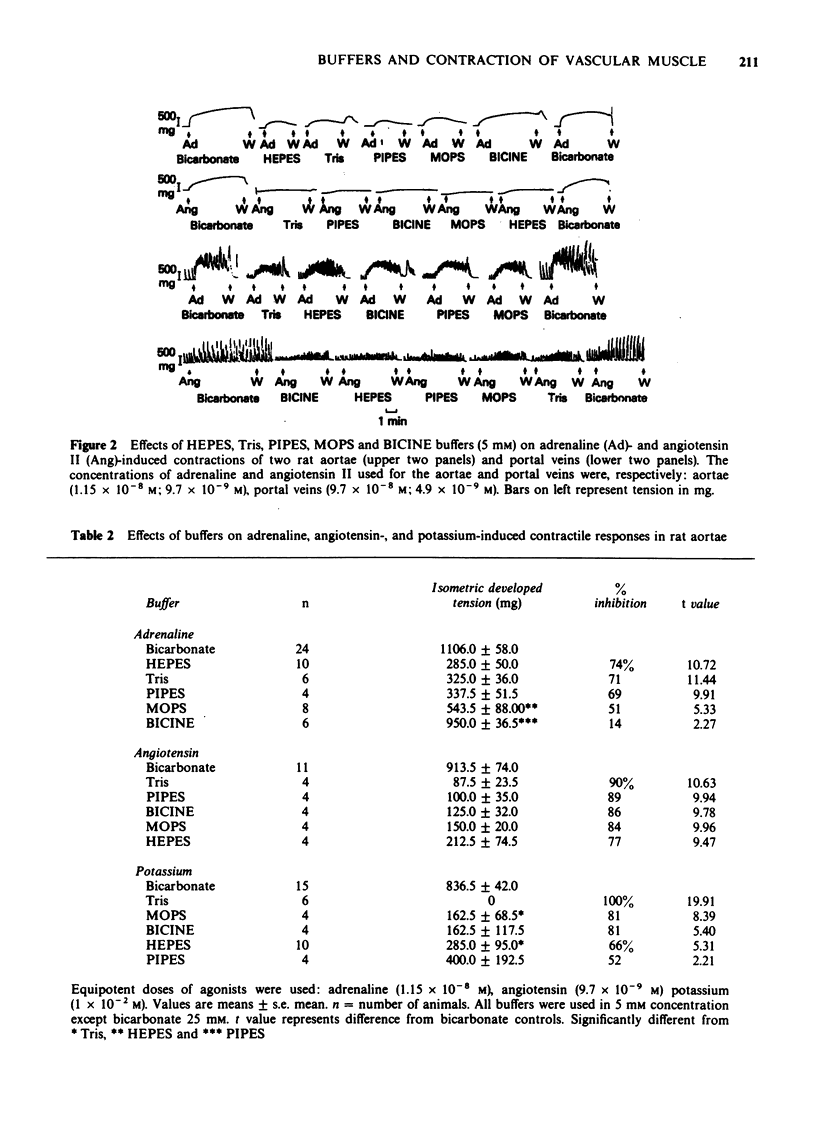
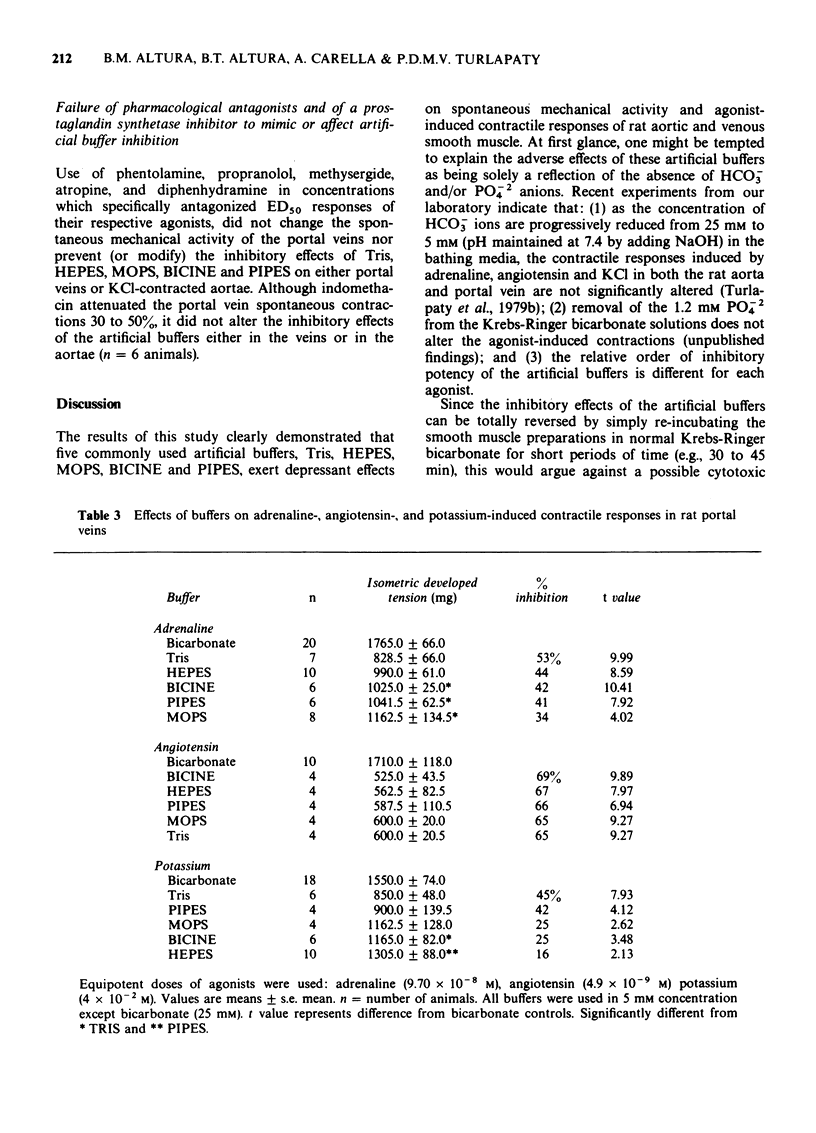


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altura B. M., Altura B. T. Differential effects of substrate depletion on drug-induced contractions of rabbit aorta. Am J Physiol. 1970 Dec;219(6):1698–1705. doi: 10.1152/ajplegacy.1970.219.6.1698. [DOI] [PubMed] [Google Scholar]
- Altura B. M., Altura B. T. Magnesium and contraction of arterial smooth muscle. Microvasc Res. 1974 Mar;7(2):145–155. doi: 10.1016/0026-2862(74)90001-6. [DOI] [PubMed] [Google Scholar]
- Altura B. T., Altura B. M. Pentobarbital and contraction of vascular smooth muscle. Am J Physiol. 1975 Dec;229(6):1635–1640. doi: 10.1152/ajplegacy.1975.229.6.1635. [DOI] [PubMed] [Google Scholar]
- Davidoff R. A., Sears E. S. Effects of synthetic buffers on reflexes in the isolated frog spinal cord. Am J Physiol. 1975 Sep;229(3):831–837. doi: 10.1152/ajplegacy.1975.229.3.831. [DOI] [PubMed] [Google Scholar]
- Dresel P. E. Negative cardiac inotropic effects of calcium ion and of N-2-hydroxyethylpiperazine-N' -2-ethanesulfonic acid (HEPES) buffer at normal and low sodium concentration. Can J Physiol Pharmacol. 1974 Oct;52(5):1025–1029. doi: 10.1139/y74-134. [DOI] [PubMed] [Google Scholar]
- Farrance M. L., Vincenzi F. F. (Ca-Mg)ATPase activity of human erythrocyte membranes: influence of incubation buffer. Experientia. 1977 Jul 15;33(7):865–866. doi: 10.1007/BF01951248. [DOI] [PubMed] [Google Scholar]
- Favalli L., Chiari M. C., Rozza A., Piccinini F. On the validity of the experimental conditions used for the study of the calcium turnover in isolated rat tail artery by the "lanthanum method". Pharmacol Res Commun. 1977 Feb;9(2):165–172. doi: 10.1016/s0031-6989(77)80019-2. [DOI] [PubMed] [Google Scholar]
- Fowler C. J., Callingham B. A., Houslay M. D. The effect of tris buffers on rat liver mitochondrial monoamine oxidase. J Pharm Pharmacol. 1977 Jul;29(7):411–415. doi: 10.1111/j.2042-7158.1977.tb11355.x. [DOI] [PubMed] [Google Scholar]
- Gillespie J. S., McKnight A. T. Adverse effects of tris hydrochloride, a commonly used buffer in physiological media. J Physiol. 1976 Jul;259(2):561–573. doi: 10.1113/jphysiol.1976.sp011482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Hanlon D. P., Watt D. S., Westhead E. W. The interaction of divalent metal ions with tris buffer in dilute solution. Anal Biochem. 1966 Aug;16(2):225–233. doi: 10.1016/0003-2697(66)90150-3. [DOI] [PubMed] [Google Scholar]
- Lorković H. Antagonism between calcium and monovalent cations in depolarized denervated muscles. Am J Physiol. 1972 Jun;222(6):1427–1434. doi: 10.1152/ajplegacy.1972.222.6.1427. [DOI] [PubMed] [Google Scholar]
- Medzon E. L., Gedies A. Substitution of 4-(2-hydroxyethyl)-1-piperazineethane sulfonic acid (HEPES) for bicarbonate in protein-free animal cell culture medium: application to vaccinia virus quantitation and fluorogenic acetylesterase assay in living LM cells. Can J Microbiol. 1971 May;17(5):651–653. doi: 10.1139/m71-105. [DOI] [PubMed] [Google Scholar]
- Morris J. E. Modifications of cell behavior and enzyme induction by zwitterionic buffers. Biochem Biophys Res Commun. 1971 Jun 18;43(6):1436–1442. doi: 10.1016/s0006-291x(71)80035-9. [DOI] [PubMed] [Google Scholar]
- TALSO P. J., CUTILLETTA A. F. THE EFFECTS OF TRIS (HYDROXYMETHYL) AMINOMETHANE ON THE COMPOSITION OF EXTRACELLULAR FLUID, SKELETAL MUSCLE, AND CARDIAC MUSCLE. Clin Pharmacol Ther. 1965 Jul-Aug;6:448–453. doi: 10.1002/cpt196564448. [DOI] [PubMed] [Google Scholar]
- Turlapaty P. D., Altura B. T., Altura B. M. Influence of tris on contracile responses of isolated rat aorta and portal vein. Am J Physiol. 1978 Aug;235(2):H208–H213. doi: 10.1152/ajpheart.1978.235.2.H208. [DOI] [PubMed] [Google Scholar]
- Turlapaty P. D., Altura B. T., Altura B. M. Interactions of Tris buffer and ethanol on agonist-induced responses of vascular smooth muscle and on calcium-45 uptake. J Pharmacol Exp Ther. 1979 Oct;211(1):59–67. [PubMed] [Google Scholar]
- Turlapaty P. D., Altura B. T., Altura B. M. Tris(hydroxymethyl)aminomethane inhibits calcium uptake in vascular smooth muscle. Biochim Biophys Acta. 1979 Mar 8;551(2):459–462. doi: 10.1016/0005-2736(89)90021-7. [DOI] [PubMed] [Google Scholar]
- Wilson W. A., Clark M. T., Pellmar T. C. Tris buffer attenuates acetylcholine responses in Aplysia neurons. Science. 1977 Apr 22;196(4288):440–441. doi: 10.1126/science.15317. [DOI] [PubMed] [Google Scholar]


