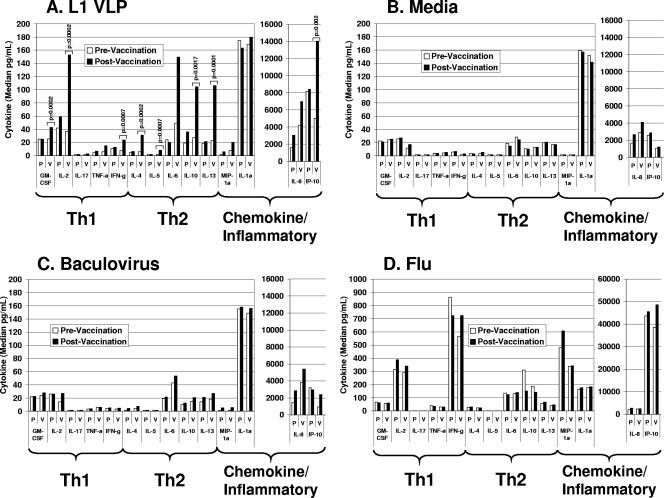
FIG. 1.
Cytokine profiles in supernatants of treated PBMCs collected before and after vaccination among L1 VLP (V; n = 19) and placebo (P; n = 7) recipients. Supernatants were tested for cytokine content using multiplex cytokine analysis as described in the text. Results are presented as median cytokine levels in picograms per milliliter. Only cytokines that exhibited a statistically significant increase in response to L1 VLP before adjusting for multiple comparisons are shown. Only significant P values are indicated. A nonparametric Wilcoxon test was used to determine statistical significance. (A) Cells from vaccine and placebo recipients were treated with HPV16 L1 VLP (2.5 μg/ml) for 72 h. P values shown were obtained from the comparisons between responses to L1 VLP from pre- and postvaccination samples and adjusted for multiple comparisons. A P value of <0.0025 was considered significant for the primary hypothesis. (B) Cells from vaccine and placebo recipients were left untreated (media) for 72 h. (C) Cells from vaccine and placebo recipients were incubated with baculovirus extract (0.1 μg/ml) for 72 h. (D) Cells from vaccine and placebo recipients were incubated with flu (1:100) for 72 h.