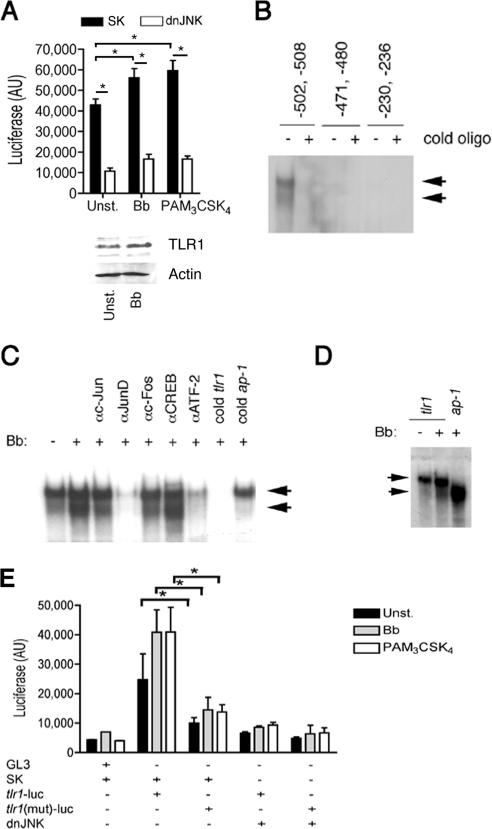
FIG. 5.
JNK1 regulates tlr1 promoter activity. (A) (Top) RAW264.7 cells were cotransfected with a plasmid containing the luciferase gene downstream of the 1-kb proximal tlr1 promoter and the dnJNK plasmid or a plasmid control (SK) and stimulated with a B. burgdorferi lysate (Bb) or PAM3-CSK4 or left unstimulated (Unst.). Luciferase activity (AU, arbitrary units) was measured after 16 h of stimulation. The data presented correspond to the average plus standard deviation of triplicate determinations of one of four experiments performed. *, P < 0.05. (Bottom) Western blot of RAW264.7 cells stimulated with 10 μg/ml of a B. burgdorferi lysate for 16 h using anti-TLR1 Ab. Equal protein input was assessed with anti-Actin Ab. (B) EMSA showing nuclear extract binding to oligonucleotides (oligo) containing putative AP-1 binding sites in the proximal 1-kb tlr1 promoter. The reactions were performed in the absence or presence of an excess (100×) of unlabeled (cold) oligonucleotides. (C) EMSA of nuclear extracts of RAW264.7 cells unstimulated and stimulated with a B. burgdorferi lysate for 30 min. The reactions were performed in the absence or the presence of Abs against c-Jun, JunD, c-Fos, CREB, and ATF-2 and in the absence or presence of unlabeled (cold) oligonucleotides corresponding to the tlr1 promoter or the AP-1 consensus binding sequence. The arrows indicate the constitutive (top) and B. burgdorferi-enhanced (bottom) complexes formed. (D) EMSA of tlr1-derived and consensus AP-1 binding oligonucleotides with nuclear extracts from unstimulated or B. burgdorferi-stimulated RAW264.7 cells. (E) RAW264.7 cells were cotransfected with plasmids containing tlr1- or tlr1Δ(−502,−508) [tlr1(mut)]-luciferase and a plasmid containing dnJNK or a plasmid control (SK). After 48 h, the cells were stimulated with a B. burgdorferi lysate (Bb) or PAM3-CSK4 or left unstimulated (Unst.). Luciferase activity was measured 16 h poststimulation. The results shown are the average plus standard error of three independent experiments. *, P < 0.05.