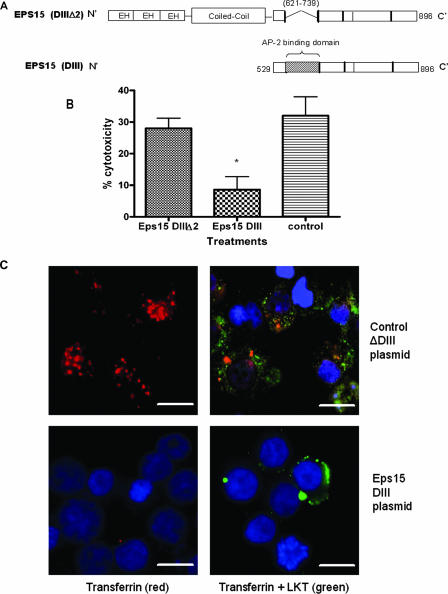
FIG. 6.
Expression of a dominant negative Eps15 protein in BL-3 cells inhibits clathrin-dependent internalization of LKT and LKT-mediated cytotoxicity. Panel A is a schematic representation of the dominant negative mutant Eps15 protein (DIII) and the control Eps15 protein (DIIIΔ2) expressed in BL-3 cells. In the Eps15 (DIII) construct, the Eps15 homology domains (EH) and N-terminal coiled coil domains that are required for clathrin coat assembly have been deleted. In panel B, BL-3 cells were transfected with 6 μg of Eps15 DIII or the control Eps15 DIII Δ2 plasmid for 6 h at 37°C and then incubated with LKT (0.2 U) for 1 h at 37°C. Cytotoxicity was measured as described previously. The data are the means and standard errors of the means of three separate experiments. The asterisk indicates that the P value is <0.05. In panel C, BL-3 cells transfected with the Eps15 DIII or Eps15 DIII Δ2 plasmid were stained for cytoplasmic LKT and for transferrin (which is internalized by the clathrin-mediated pathway). BL-3 cells incubated with LKT were fixed, permeabilized, blocked with 3% bovine serum albumin, and incubated with FITC-conjugated anti-LKT MAb and rhodamine-conjugated transferrin. Images were visualized by confocal microscopy. The control plasmid-transfected BL-3 cells demonstrated internalization of transferrin (red) and LKT (green), while the Eps15 DIII plasmid-transfected BL-3 cells showed reduced internalization of LKT and virtually no signal from transferrin. Cells were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI) nuclear stain (blue). The images in panel C show representative cells from three separate experiments that were performed. Bars = 10 μm.