Abstract
In previous studies we showed that a Cryptococcus neoformans mutant lacking glucosylceramide (Δgcs1) is avirulent and unable to reach the brain when it is administered intranasally into an immunocompetent mouse and is contained in a lung granuloma. To determine whether granuloma formation is key for containment of C. neoformans Δgcs1, we studied the role of C. neoformans glucosylceramide in a T- and NK-cell-immunodeficient mouse model (Tgɛ26) in which alveolar macrophages (AMs) are not activated and granuloma formation is not expected. The results show that Tgɛ26 mice infected with Δgcs1 do not produce a lung granuloma and that the Δgcs1 mutant proliferates in the lungs and does disseminate to the brain, although its virulence phenotype is dramatically reduced. Since Δgcs1 can grow only in acidic niches, such as the phagolysosome of AMs, and not in neutral or alkaline environments, such as the extracellular spaces, we hypothesize that in immunodeficient mice Δgcs1 proliferates inside AMs. Indeed, we found that depletion of AMs significantly improved Tgɛ26 mouse survival and decreased the dissemination of Δgcs1 cells to the central nervous system. Thus, these results suggest that the growth of Δgcs1 in immunodeficient mice is maintained within AMs. This study highlights the hypothesis that AMs may exacerbate C. neoformans infection in conditions in which there is severe host immunodeficiency.
Cryptococcus neoformans is an environmental pathogenic yeast which causes life-threatening fungal meningoencephalitis in immunocompromised as well as immunocompetent hosts (2, 17, 22, 25). After inhalation of desiccated yeast cells or spores, the lung provides niches for intracellular and extracellular growth. Thus, C. neoformans is a facultative intracellular pathogen. If uncontained or uncontrolled, fungal cells can travel to other organs, particularly the brain, where the disease is difficult to treat. A better understanding of factors that augment the ability of the fungus to grow in the lung environment is necessary to contain the disease before it disseminates to cause systemic infection.
Following inhalation, alveolar macrophages (AMs) represent the first line of defense against inhaled microorganisms, such as C. neoformans, by providing a means for their removal and destruction (19). AMs are critical in initiating a specific cell-mediated response through antigen presentation, costimulation of T cells, and cytokine and chemokine release. Once activated, macrophages serve as effector cells contributing to the control of the infection and the formation of granulomas. Thus, in conditions in which there is depressed T-cell-mediated immunity (e.g., patients with AIDS, lymphoma, and sarcoidosis and subjects taking immunosuppressive medications, such as corticosteroids, cyclophosphamide, or azathioprine), cryptococcosis is more prevalent due to the inability of the host to control the infection.
In addition, since C. neoformans is a facultative intracellular pathogen, it can replicate within macrophages (7, 10), specifically within their phagolysosomes (9, 14). Thus, although development of cryptococcosis is due to both intra- and extracellular C. neoformans growth (3, 8, 11, 15, 30), it is reasonable to hypothesize that in conditions in which there is immunodeficiency, the intracellular component could exacerbate cryptococcosis. This hypothesis is derived from the following observations: (i) C. neoformans can replicate faster intracellularly than extracellularly (8); (ii) C. neoformans can disseminate within macrophages (5, 16); and (iii) live C. neoformans cells can escape from macrophages without killing the host cells (1, 18). Therefore, if upon phagocytosis AMs cannot effectively kill C. neoformans, phagocytosis can be considered an opportunity for the fungus to produce disease. Thus, the study of genes and factors of the pathogen or host that contribute to intra- or extracellular growth should be useful for development of novel strategies for prevention and treatment of fungal infections.
Recently, we identified a fungal glycosphingolipid called glucosylceramide (GlcCer) that is required for C. neoformans to cause a brain infection when the fungus is introduced intranasally into an immunocompetent mouse model (CBA/J). Particularly in CBA/J mice, the Δgcs1 mutant is almost totally contained within a lung granuloma (23). Thus, we wondered whether this particular phenotype was due to the presence of AMs and the subsequent formation of the granuloma and to a unique characteristic of Δgcs1 cells, that they are able to grow within AMs and not in alveolar spaces.
We reasoned that in a host unable to form a granuloma, the phagocytosed Δgcs1 mutant would proliferate within nonactivated macrophages and eventually kill the host. To address this hypothesis, we studied the pathogenesis of Δgcs1 in an immunodeficient transgenic mouse model that lacks both T and NK cells isogenic to CBA/J cells (32, 33) and determined the role of AMs of these mice during the course of C. neoformans infection.
MATERIALS AND METHODS
Strains, medium, and reagents.
C. neoformans var. grubii serotype A H99-derived strains Δgcs1 and Δgcs1+GCS1 have been described previously in great detail (23). H99 (wild type [WT]) and H99-derived strains Δgcs1 and Δgcs1+GCS1 were grown in yeast extract-peptone-2% dextrose (YPD) medium from Difco. The YPD medium plates used were supplemented with chloramphenicol (100 μg/ml) and ampicillin (100 μg/ml). All reagents used were obtained from Sigma Chemical Co. (St. Louis, MO) unless otherwise specified.
Animal studies.
Four- to six-week-old Tgɛ26 mice (32, 34) from the Animal Core Facility, Medical University of South Carolina, Charleston, were used for this study. The Tgɛ26 mice are isogenic to CBA/J mice (32, 34). Mice were anesthetized by intraperitoneal injection of 60 μl of a xylaxine-ketamine mixture containing 5 mg xylazine and 95 mg ketamine per kg of body weight. Wild-type and mutant strains of C. neoformans were grown in YPD medium for 24 h at 30°C. The yeast cells were harvested, washed three times by centrifugation at 3,000 rpm for 10 min, and resuspended in sterile phosphate-buffered saline (PBS) (pH 7.4) at a concentration of 2.5 × 107 cells/ml. For each group 10 mice were infected intranasally with 20 μl containing 5 × 105 WT, Δgcs1, or Δgcs1+GCS1 cells. Mice were fed ad libitum and monitored by inspection twice a day. Mice that appeared to be moribund or in pain or to have clinical signs of meningoencephalitis were sacrificed using CO2 inhalation followed by cervical dislocation. All animal procedures were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee and followed the guidelines of the American Veterinary Medical Association. Survival data from the mouse experiment were analyzed by the Kruskal-Wallis test. A P value of less than 0.05 was considered significant.
AM depletion.
Liposomes containing clodronate (dichloromethylenediphosphonic acid, disodium salt) and empty liposomes used as a control were prepared as described previously (31). Briefly, 8 mg cholesterol (Avanti Polar Lipids) and 86 mg l-α-phosphatidylcholine (Avanti Polar Lipids) were dissolved in chloroform, which was then slowly evaporated. The thin, filmy layer was resuspended in 10 ml of PBS or 0.6 M clodronate. The mixture was exposed to N2 gas and incubated for 2 h at room temperature with gentle shaking. The mixture was then sonicated and incubated for another 2 h to allow liposome swelling. The solution was centrifuged at 10,000 × g for 15 min, and the liposomes containing clodronate were collected and washed twice with sterile PBS. The liposomes were kept at 4°C under N2 until use for a maximum of 2 weeks. AM depletion was evaluated by intranasally introducing 60 μl of PBS, PBS-containing liposomes, or liposomes containing clodronate into mice and performing bronchoalveolar lavage after 48 h or 7 days. Bronchoalveolar lavage was done by inserting a stub adapter into the trachea of a mouse and rinsing the alveolar spaces 10 times using 0.5 ml PBS (pH 7.4) for each wash. The collected fluid was spun down, and viable AMs were quantified via trypan blue exclusion. Lavage samples were taken from two mice per time point per group. The experimental groups used for survival studies consisted of five mice each receiving PBS (control group), PBS-containing liposomes (control group), or clodronate-containing liposomes. Two days before infection with yeast cells, the mice were anesthetized and given 60 μl of PBS, PBS-containing liposomes, or clodronate-containing liposomes. After 48 h, mice were infected with the yeast cells as described above. Mice continued to receive PBS, PBS-containing liposome, or clodronate-containing liposome treatment weekly.
Tissue fungal burden.
The lungs, brain, spleen, and kidneys were removed aseptically from mice on days 4, 8, and 12 for WT-infected mice and on days 16, 26, 36, 50, 64, and 70 for Δgcs1-infected mice (although only the mice treated with clodronate were able to survive until the last two time points). Three mice for each time point were used. The organs were homogenized in sterile PBS using a Stomacher 80 (Lab System, Fisher Scientific, Pittsburgh, PA) for 2 min. Serial dilutions were then plated onto YPD medium plates, CFU were counted, and the data were recorded. Statistical analysis was performed using Student's t test, and a P value of <0.05 was considered significant.
Histological analyses.
Organs were harvested, fixed for 48 h in 37% formaldehyde, paraffin embedded, sectioned, and stained with movat, mucicarmine, or hematoxylin and eosin as described previously (23).
RESULTS
Virulence of C. neoformans Δgcs1 in mouse models.
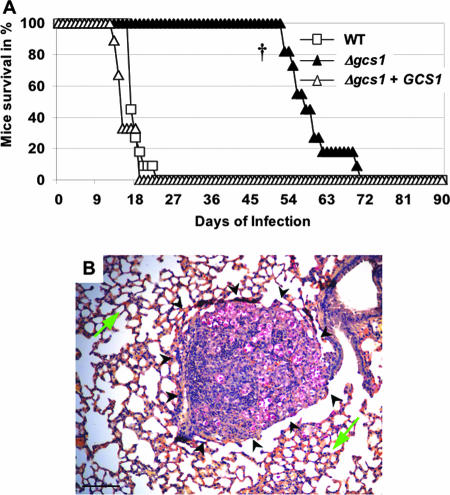
In previous studies, we showed that a mutant lacking GlcCer (Δgcs1) is avirulent in an immunocompetent mouse model (CBA/J mice) when mice are challenged intranasally with Δgcs1 (23). The average survival times of CBA/J mice infected with the WT and with Δgcs1+GCS1 were 24.6 ± 3.9 and 27.3 ± 4.8 days, respectively, whereas the mice infected with C. neoformans Δgcs1 were all alive at day 90 (P < 0.0001). Interestingly, mice infected with Δgcs1 did not eradicate the C. neoformans infection; instead, they contained it in the lung tissue with the formation of several granulomas (23).
In order to address whether the formation of granulomas is key for the containment of C. neoformans Δgcs1, we performed virulence studies with an isogenic mouse model in which NK and T cells are deficient (Tgɛ26 mice). In these mice, AMs are not activated through a gamma interferon-mediated mechanism (26), and classical granuloma formation is not expected. The average survival times of Tgɛ26 mice infected with the WT and with Δgcs1+GCS1 were 17.2 ± 1.9 and 14.9 ± 2.4 days, respectively, whereas the average survival time of mice infected with the C. neoformans Δgcs1 mutant was 58.5 ± 6.2 days (P < 0.001) (Fig. 1A). As expected, lungs of Tgɛ26 mice infected with Δgcs1 did not show any classical signs of granuloma formation (e.g., deposition of fibrotic and collagen tissues surrounding macrophages with necrotic tissues inside). Instead, there were several nodular structures characterized by infiltration of C. neoformans Δgcs1 cells with macrophages and granulocytes (Fig. 1B). These results suggest that the absence of granulomas renders mice susceptible to Δgcs1 infection.
FIG. 1.
Virulence studies with C. neoformans WT and Δgcs1 and reconstituted strains in Tgɛ26 mice, which are isogenic to CBA/J mice. (A) Mouse survival. The average survival times for Tgɛ26 mice infected with WT or Δgcs1+GCS1 were 17.2 ± 1.9 and 14.9 ± 2.4 days, respectively. Conversely, the Δgcs1-infected mice survived 58.5 ± 6.2 days (P < 0.001 †). (B) Mucicarmine staining of a lung obtained from a Tgɛ26 mouse infected with C. neoformans Δgcs1 for 36 days. Fungal cells (red) are contained within a nodular structure (black arrowheads) with a macrophage and granulocyte infiltration. The green arrow indicates normal lung tissue. Bar = 50 μm.
Pharmacological depletion of AMs.
In order to study the role of the inactivated macrophages during the course of infection, the effect of depletion of AMs was determined. First, the efficacy of the treatment was studied, and then its effect on the infection was studied. Sixty microliters of a liposome suspension of clodronate was intranasally injected into each Tgɛ26 mouse. Two control groups were used; one group received 60 μl of PBS-containing liposomes, and the other received 60 μl of PBS. At 48 h or 7 days after injection, mice were sacrificed, the bronchoalveolar lavage fluid was collected, and the viability of AMs was assessed microscopically via trypan blue exclusion. After 48 h, clodronate-treated mice showed a significant decrease (58%) in the number of AMs compared to mice treated with PBS or PBS-containing liposomes (P < 0.05). After 7 days, clodronate-treated mice showed a 52% reduction in the number of AMs compared to control-treated mice (P < 0.05). In a pilot experiment performed for previous studies (28), we checked if the low number of AMs was maintained in an uninfected mouse for a long period using a single weekly administration of clodronate. We found that weekly administration of clodronate maintained the number of AMs at a low level (∼50%) up to 42 days after the first administration (data not shown). These results showed that administration of a single dose of clodronate-containing liposomes is sufficient to decrease the number of AMs by 50% within 48 h, that this decrease is maintained for at least 7 days, and that weekly additional administration is sufficient to maintain a low number of AMs for 7 weeks. These results are consistent with previously reported observations (27) and with the fact that complete regeneration of AMs in mice requires 18 days (29).
Effect of macrophage depletion on survival of C. neoformans-infected Tgɛ26 mice.
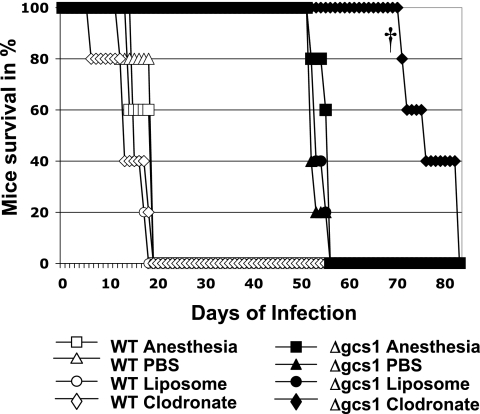
After 48 h of clodronate or control treatment, mice received an intranasal injection of the C. neoformans WT or Δgcs1 mutant, as described in Materials and Methods. In addition, since mice were subjected to repeated anesthesia because of the weekly administration of liposomes or PBS, an additional group of infected mice receiving only weekly anesthesia without liposome or drug injection was included as a control for anesthesia. The average survival times of C. neoformans-infected mice receiving anesthesia alone, the PBS control, empty liposomes, and clodronate were 16 ± 2.7, 16.8 ± 2.7, 15 ± 1.4, and 12.8 ± 5.2 days, respectively (Fig. 2). Overall, clodronate-treated mice infected with the C. neoformans WT did not show a significant change in survival compared to the control groups. On the other hand, the average survival time of Δgcs1-infected mice receiving clodronate was significantly increased (76.6 ± 5.3 days) compared to the average survival times observed for Δgcs1-infected mice receiving anesthesia alone, the PBS control, and empty liposomes (54 ± 1.7, 52.8 ± 1.3, and 52.8 ± 1.6 days, respectively) (P < 0.05) (Fig. 2). These results showed that Δgcs1-infected mice with fewer AMs survived significantly longer than mice having a normal number of AMs, suggesting that these phagocytic cells may enhance the progression of disease by favoring the development and dissemination of Δgcs1 cells in Tgɛ26 mice.
FIG. 2.
Survival studies with Tgɛ26 mice receiving weekly doses of anesthesia alone, PBS, PBS-containing liposomes, or clodronate-containing liposomes. The mice were infected with C. neoformans WT or Δgcs1. While there is no significant difference among the different groups infected with C. neoformans WT, clodronate-treated mice survived an average of 76.6 ± 5.3 days (P < 0.05 †), which is significantly longer than the controls.
Tissue burden studies.
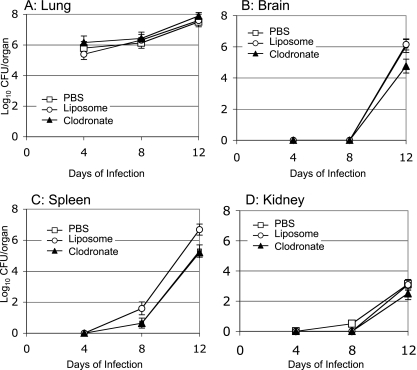
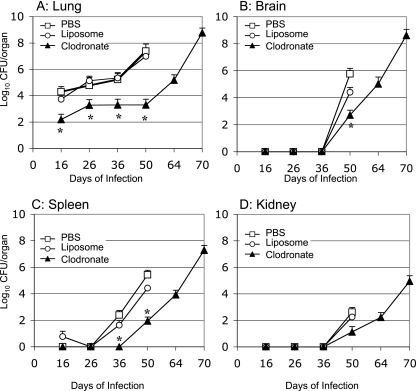
To address the effect of macrophage depletion on the dissemination of Δgcs1 cells, we performed tissue burden studies of lungs, brains, spleens, and kidneys after infection with the C. neoformans WT or Δgcs1 strain. No statistically significant differences were found in tissue colonization by C. neoformans WT cells in all organs analyzed among the infected groups that received PBS or empty or clodronate-containing liposomes (Fig. 3). Interestingly, mice infected with the Δgcs1 mutant treated with clodronate had a significantly lower number of fungal cells in all organs analyzed compared to the numbers in control-treated groups (Fig. 4A to D) (P < 0.05). Of interest, lungs infected with the Δgcs1 mutant showed an early decrease in fungal cell number in all three experimental groups (Fig. 4A), a result that was also obtained in our previous study performed with the isogenic immunocompetent CBA/J mice (see Fig. S1 in the supplemental material) (23). This decrease in the fungal load is attributed to the death or clearance of fungal cells not able to grow in the lung extracellular environment (such as the alveolar spaces). More interesting is the dramatic decrease in the number of Δgcs1 cells in the macrophage-depleted Tgɛ26 lungs (Fig. 4A). It is remarkable that a ∼50% decrease in the number of AMs reduced the lung Δgcs1 fungal load >3,000-fold (P < 0.001 for a comparison of clodronate- and liposome-treated mice). Eventually, the Δgcs1 cells did seem to grow in the lungs, although a significant delay was clearly observed in the macrophage-depleted mice. Similarly, in all other organs there seemed to be delays ranging from approximately 14 to 28 days in fungal growth in the clodronate-treated mice compared to the control-treated mice. These results suggest that depletion of AMs significantly delays the growth of Δgcs1 cells in the lungs and other organs of Tgɛ26 immunocompromised mice.
FIG. 3.
Viable C. neoformans in internal organs of Tgɛ26 mice that were treated with PBS, PBS-containing liposomes, or clodronate-containing liposomes and infected intranasally with C. neoformans WT. No significant differences in the number of CFU were observed in lung (A), spleen (C), or kidney (D) homogenates at different time points after intranasal infection with C. neoformans WT. Clodronate-treated mice showed reductions in the tissue burden of C. neoformans WT in the brain (B) and in the spleen (C), but the differences were not statistically significant. The results are expressed as CFU per organ on a logarithmic scale.
FIG. 4.
Viable C. neoformans in internal organs of Tgɛ26 mice that were treated with PBS, PBS-containing liposomes, or clodronate-containing liposomes and infected intranasally with C. neoformans Δgcs1. Growth and dissemination of the Δgcs1 strain in the lungs (A), brain (B), kidneys (C), and spleen (D) were retarded in the clodronate-treated group. An asterisk indicates that the P value is <0.05 for a comparison of clodronate- and liposome-treated mice. No significant differences were observed when liposome- and PBS-treated mice were compared.
Histopathology.
To gain further insight into the host inflammatory response and the localization of C. neoformans Δgcs1 cells in lungs, we performed a histology analysis of lungs of Tgɛ26 mice infected with the Δgcs1 strain and receiving liposomes containing PBS or clodronate. At early time points (day 16 of infection), in lungs receiving empty liposomes Δgcs1 cells were found predominantly intracellularly in the lung parenchyma (Fig. 5A and 5B) or in lymph nodes (data not shown), and the host inflammatory response was moderate or absent. As the infection progressed, at day 36 the number of Δgcs1 cells increased in mice treated with empty liposomes and there was moderate infiltration of macrophages and granulocytes (see Fig. S2A in the supplemental material), and by day 50 of infection Δgcs1 cells had significantly disrupted the lung structure and infiltrated the lymph nodes, with destruction of the tissue (see Fig. S2C in the supplemental material).
FIG. 5.
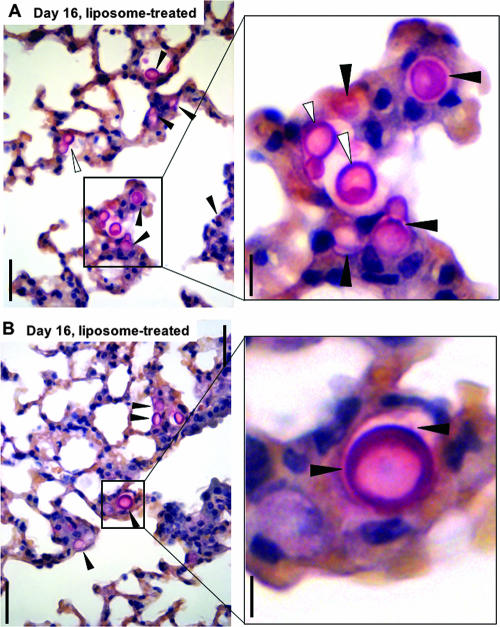
Histopathology of mucicarmine-stained Tgɛ26 lungs infected with the Δgcs1 strain and treated with empty liposomes. Panels A and B show lung fields from two different mice at day 16 of infection. The filled arrowheads indicate Δgcs1 cells within macrophages, whereas the open arrowheads indicate extracellular Δgcs1 cells. Bars = 50 μm (left panels) and 10 μm (right panels).
In contrast, in the lungs of mice treated with clodronate there were few Δgcs1 cells in the lung parenchyma at day 16 (Fig. 6A, and 6B), and these fungal cells were mostly in the extracellular environment. At day 36 of infection limited granulocyte infiltrations with very few Δgcs1 cells were observed (see Fig. S2B in the supplemental material). At day 70 of infection, Δgcs1 cells appeared to be present preferentially in the lung parenchyma (see Fig. S2D in the supplemental material). Overall, lungs treated with clodronate and infected with the Δgcs1 strain were characterized by a limited inflammatory response and by a lack of lymph node infiltration (data not shown).
FIG. 6.
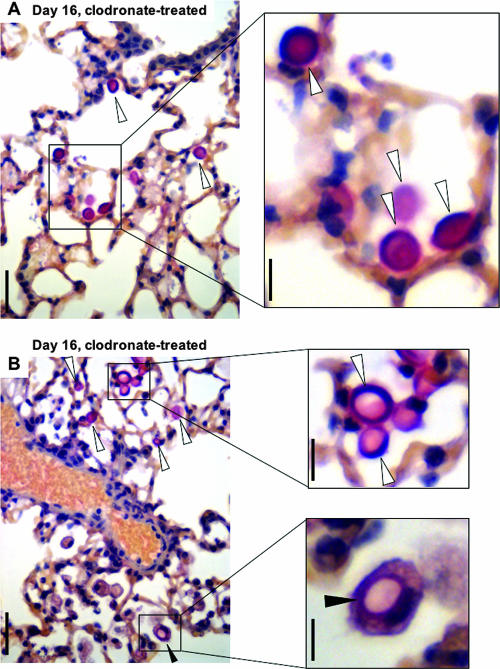
Histopathology of mucicarmine-stained Tgɛ26 lungs infected with the Δgcs1 strain and treated with clodronate. Panels A and B show lung fields from two different mice at day 16 of infection. The open arrowheads indicate extracellular Δgcs1 cells. The filled arrowheads in panel B indicate a Δgcs1 cell within a macrophage. Bars = 50 μm (left panels) and 10 μm (right panels).
These results showed that depletion of AMs significantly decreased the progression of cryptococcal disease caused by Δgcs1 in the absence of T and NK cells. In T-cell- and NK cell-deficient mice with depleted AMs the progression of Δgcs1 cryptococcal disease was significantly decreased compared to that in T- and NK-cell-deficient mice in which AMs were not depleted.
DISCUSSION
Previous studies in our laboratory have shown that a C. neoformans GlcCer mutant (Δgcs1) is not pathogenic and is unable to reach the central nervous system when it is inhaled by an immunocompetent mouse. We also showed that C. neoformans Δgcs1 was not able to grow in the lungs and was contained within a lung granuloma. Finally, we showed that Δgcs1 cells were in growth arrest in vitro when they were incubated at a neutral or alkaline pH with 5% CO2. Based on these observations, we hypothesized that GlcCer favors growth of C. neoformans in extracellular environments, which are characterized by a neutral or alkaline pH and 5% CO2. Even if C. neoformans Δgcs1 can grow intracellularly, the host responded with formation of a well-organized lung granuloma.
In this study we showed that under conditions in which the animal is unable to form a lung granuloma (an NK- and T-cell-deficient host), Δgcs1 eventually disseminates to the brain and kills the host. Importantly, we show that depletion of AMs significantly delays dissemination of C. neoformans Δgcs1 cells and improves mouse survival, suggesting that in animal models in which macrophages cannot be activated AMs favor fungal growth and dissemination.
C. neoformans is a facultative intracellular pathogen known to survive and replicate in extracellular environments, such as the alveolar spaces, the bloodstream, and host tissue, and within phagocytic cells, such as macrophages (7, 10). Within certain macrophages (nonactivated) C. neoformans replicates in vitro more rapidly than it replicates extracellularly. Thus, it can be hypothesized that in immunocompromised individuals, in which macrophages are less activated or not activated, C. neoformans replicates more vigorously intracellularly, where it can grow unthreatened by the compromised host cellular response. This hypothesis is supported by studies suggesting that C. neoformans disseminates within macrophages from the lung to the mediastinal area and to the brain when the host cellular response is impaired (5, 16, 27). Thus, the location of C. neoformans cells (intra- or extracellular) in an immunodeficient host may have direct consequences not only for the pathogenesis of cryptococcosis but, importantly, for the outcome of the infection. Fungal regulatory mechanisms controlling pathogen growth based on its location therefore assume critical importance for better management of the fungal disease process.
The results of our work can be summarized as follows. In the extracellular space of the lung (neutral pH and 5% CO2) the Δgcs1 cells are in growth arrest. Once they are taken up by the AMs, they are able to grow. This conclusion is supported by previous studies in which we showed that although growth in vitro of the Δgcs1 mutant in 5% CO2 at alkaline pH is arrested, it can be restored if cells are switched to low pH (see Fig. S3 in the supplemental material). Intracellular Δgcs1 cells can then be extruded outside the macrophages without killing the host cells. This hypothesis is supported by two recent studies which showed that C. neoformans cells can enter, replicate in, and be expelled by AMs without harming the macrophages (1, 18). Finding themselves in the harsh conditions of the extracellular milieu again, the Δgcs1 cells undergo growth arrest until they find refuge in other macrophages, and this cycle can be repeated, augmenting the number of Δgcs1 cells. In other words, C. neoformans Δgcs1 can be considered an “obligate intracellular pathogen.” Since upon C. neoformans extrusion macrophages are still intact, one macrophage can host several C. neoformans cells. It is also possible, however, that uncontrolled intracellular growth by the Δgcs1 mutant would lead to macrophage rupture. Regardless of the mechanism, for intracellular multiplication we hypothesize that the Δgcs1 mutant preferentially uses nonactivated macrophages throughout their lifetime.
In conditions in which the host is immunodeficient, a granuloma is not formed, and thus the large number of Δgcs1 cells growing in the lung macrophages would eventually disseminate to the brain, killing the host. This dissemination does not necessarily need to be intracellular. C. neoformans Δgcs1 can also travel extracellularly in the bloodstream. It is the extracellular replication, not the extracellular survival, that is inhibited. Indeed, ∼90% of C. neoformans Δgcs1 cells are still alive in neutral or alkaline and 5% CO2 environments after 72 h of incubation (23). Thus, it is expected that C. neoformans Δgcs1 would also survive in the bloodstream. Upon intravenous injection, C. neoformans cells may reach the brain within hours (4), and Δgcs1 cells do indeed reach the brain after intravenous injection (21, 23). Once in the brain, growth of extracellular cells can be restored in low-pH environments (e.g., within microglial cells and in brain abscesses). Indeed, a pharmacological treatment that alkalinizes the intracellular environment of microglial cells significantly decreases C. neoformans growth (20).
The observation that the survival of Tgɛ26 mice infected with Δgcs1 significantly increases when AMs are depleted clearly suggests that Δgcs1 causes disease mainly through its intracellular growth within nonactivated macrophages. It is remarkable that although Tgɛ26 mice lack T cells, NK cells, and ∼60% of the total AMs, they still survive after infection by the Δgcs1 strain for an additional 18 to 20 days. The hypothesis that nonactivated macrophages favor the dissemination of C. neoformans is also supported by the decreased dissemination of the C. neoformans WT to the brain in immunodeficient mice in which AMs are depleted (Fig. 3B), whereas a decrease in dissemination of fungal cells was not observed in previous studies in which AMs were depleted in immunocompetent mice infected with the C. neoformans WT (28). Shao et al. also showed that depletion of AMs leads to a decrease in C. neoformans proliferation in the lungs at 3 and 14 days postinfection (27). It is noteworthy that we used 40% less clodronate than Shao et al. used and yet a significant effect on fungal dissemination was obtained. Taken together, these studies clearly suggest that during C. neoformans infection AMs may be detrimental for the host in immunodeficiency conditions.
AIDS patients are the most common victims of cryptococcosis, and in these patients the CD4+ T-cell count is low and NK cells are dysfunctional. The advantage of working with Tgɛ26 immunodeficient mice is that, since they are T and NK cell deficient, they mimic the immune system of AIDS patients, although it is important to consider that human macrophages may behave differently than mouse macrophages against C. neoformans infection (6). Nevertheless, the mouse macrophage is a well-established model and very useful for gathering preliminary results that can be validated with human macrophages.
Our results may introduce two promising strategies for new treatment options when there is host immunodeficiency: C. neoformans GlcCer and C. neoformans Gcs1 as target opportunities. In particular, one could envision that targeting GlcCer (e.g., by administering an anti-GlcCer antibody or by inhibiting the Gcs1 enzyme using a drug) (24) would block extracellular growth of C. neoformans. If the anti-GlcCer treatment could then be combined with a treatment that alkalinizes the phagolysosome and facilitates the extrusion of C. neoformans cells from the intracellular milieu (1, 18) (e.g., by using chloroquine [12] or by using molecules or proteins that prevent phagocytosis [16], including administration of antiphagocytic protein 1), then C. neoformans cells would have no place to replicate. If this were done, the efficacy of current antifungal drugs (e.g., fluconazole) could also be greatly improved. For instance, although the antifungal activity of fluconazole increases when it is used in combination with chloroquine, the increase in the efficacy of the combination over that of fluconazole alone is not dramatic (12, 13). Thus, concomitant administration of an anti-GlcCer with a fluconazole-chroloquine treatment may significantly improve the outcome of C. neoformans infection. We are very excited about these possibilities.
Supplementary Material
Acknowledgments
We thank Chiara Luberto and Ed Balish for discussions. We appreciate the work of Jennifer Henry, who helped with the animal studies.
This work was supported in part by the Burroughs Wellcome Fund, the National Institutes of Health (grant AI56168 to M.D.P.), and the Centers of Biomedical Research Excellence Program of the National Center for Research Resources (grant RR17677 project 2 to M.D.P.). The animal studies were partially supported by the National Institutes of Health (grant C06 RR015455 from the Extramural Research Facilities Program of the National Center for Research Resources). J.S. was supported in part by Medical Scientist Training Grant GM08716 from the National Institutes of Health. M.D.P. is a Burroughs Wellcome New Investigator in Pathogenesis of Infectious Diseases.
Editor: A. Casadevall
Footnotes
Published ahead of print on 30 July 2007.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Alvarez, M., and A. Casadevall. 2006. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr. Biol. 16:2161-2165. [DOI] [PubMed] [Google Scholar]
- 2.Casadevall, A., and J. R. Perfect. 1998. Cryptococcus neoformans, p.381-405. ASM Press, Washington, DC.
- 3.Chang, W. L., H. C. van der Heyde, and B. S. Klein. 1998. Flow cytometric quantitation of yeast a novel technique for use in animal model work and in vitro immunologic assays. J Immunol. Methods 211:51-63. [DOI] [PubMed] [Google Scholar]
- 4.Chang, Y. C., M. F. Stins, M. J. McCaffery, G. F. Miller, D. R. Pare, T. Dam, M. Paul-Satyaseela, K. S. Kim, and K. J. Kwon-Chung. 2004. Cryptococcal yeast cells invade the central nervous system via transcellular penetration of the blood-brain barrier. Infect. Immun. 72:4985-4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chretien, F., O. Lortholary, I. Kansau, S. Neuville, F. Gray, and F. Dromer. 2002. Pathogenesis of cerebral Cryptococcus neoformans infection after fungemia. J. Infect. Dis. 186:522-530. [DOI] [PubMed] [Google Scholar]
- 6.Del Poeta, M. 2004. Role of phagocytosis in the virulence of Cryptococcus neoformans. Eukaryot. Cell 3:1067-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diamond, R. D., and J. E. Bennett. 1973. Disseminated cryptococcosis in man: decreased lymphocyte transformation in response to Cryptococcus neoformans. J. Infect. Dis. 127:694-697. [DOI] [PubMed] [Google Scholar]
- 8.Diamond, R. D., and J. E. Bennett. 1973. Growth of Cryptococcus neoformans within human macrophages in vitro. Infect. Immun. 7:231-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldmesser, M., Y. Kress, P. Novikoff, and A. Casadevall. 2000. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect. Immun. 68:4225-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldmesser, M., S. Tucker, and A. Casadevall. 2001. Intracellular parasitism of macrophages by Cryptococcus neoformans. Trends Microbiol. 9:273-278. [DOI] [PubMed] [Google Scholar]
- 11.Huffnagle, G. B., M. B. Boyd, N. E. Street, and M. F. Lipscomb. 1998. IL-5 is required for eosinophil recruitment, crystal deposition, and mononuclear cell recruitment during a pulmonary Cryptococcus neoformans infection in genetically susceptible mice (C57BL/6). J. Immunol. 160:2393-2400. [PubMed] [Google Scholar]
- 12.Khan, M. A., R. Jabeen, and O. Mohammad. 2004. Prophylactic role of liposomized chloroquine against murine cryptococcosis less susceptible to fluconazole. Pharm. Res. 21:2207-2212. [DOI] [PubMed] [Google Scholar]
- 13.Khan, M. A., R. Jabeen, T. H. Nasti, and O. Mohammad. 2005. Enhanced anticryptococcal activity of chloroquine in phosphatidylserine-containing liposomes in a murine model. J. Antimicrob. Chemother. 55:223-228. [DOI] [PubMed] [Google Scholar]
- 14.Levitz, S. M. 2001. Cryptococcus neoformans: intracellular or extracellular? Trends Microbiol. 9:417-418. [DOI] [PubMed] [Google Scholar]
- 15.Levitz, S. M., S. H. Nong, K. F. Seetoo, T. S. Harrison, R. A. Speizer, and E. R. Simons. 1999. Cryptococcus neoformans resides in an acidic phagolysosome of human macrophages. Infect. Immun. 67:885-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luberto, C., B. Martinez-Marino, D. Taraskiewicz, B. Bolanos, P. Chitano, D. L. Toffaletti, G. M. Cox, J. R. Perfect, Y. A. Hannun, E. Balish, and M. Del Poeta. 2003. Identification of App1 as a regulator of phagocytosis and virulence of Cryptococcus neoformans. J. Clin. Investig. 112:1080-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lui, G., N. Lee, M. Ip, K. W. Choi, Y. K. Tso, E. Lam, S. Chau, R. Lai, and C. S. Cockram. 2006. Cryptococcosis in apparently immunocompetent patients. QJM 99:143-151. [DOI] [PubMed] [Google Scholar]
- 18.Ma, H., J. E. Croudace, D. A. Lammas, and R. C. May. 2006. Expulsion of live pathogenic yeast by macrophages. Curr. Biol. 16:2156-2160. [DOI] [PubMed] [Google Scholar]
- 19.Mansour, M. K., and S. M. Levitz. 2002. Interactions of fungi with phagocytes. Curr. Opin. Microbiol. 5:359-365. [DOI] [PubMed] [Google Scholar]
- 20.Mazzolla, R., R. Barluzzi, A. Brozzetti, J. R. Boelaert, T. Luna, S. Saleppico, F. Bistoni, and E. Blasi. 1997. Enhanced resistance to Cryptococcus neoformans infection induced by chloroquine in a murine model of meningoencephalitis. Antimicrob. Agents Chemother. 41:802-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell, A. P. 2006. Cryptococcal virulence: beyond the usual suspects. J. Clin. Investig. 116:1481-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perfect, J. R. 2006. Cryptococcus neoformans: a sugar-coated killer, p.281-303. In J. Heitman, S. G. Filler, J. E. Edwards, Jr., and A. P. Mitchell (ed.), Molecular principles of fungal pathogenesis,vol. 1. ASM Press, Washington, DC. [Google Scholar]
- 23.Rittershaus, P. C., T. B. Kechichian, J. Allegood, A. H. J. Merrill, M. Hennig, C. Luberto, and M. Del Poeta. 2006. Glucosylceramide is an essential regulator of pathogenicity of Cryptococcus neoformans. J. Clin. Investig. 116:1651-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodrigues, M. L., L. R. Travassos, K. R. Miranda, A. J. Franzen, S. Rozental, W. de Souza, C. S. Alviano, and E. Barreto-Bergter. 2000. Human antibodies against a purified glucosylceramide from Cryptococcus neoformans inhibit cell budding and fungal growth. Infect. Immun. 68:7049-7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rozenbaum, R., and A. J. Goncalves. 1994. Clinical epidemiological study of 171 cases of cryptococcosis. Clin. Infect. Dis. 18:369-380. [DOI] [PubMed] [Google Scholar]
- 26.Schofield, D. A., C. Westwater, and E. Balish. 2004. Beta-defensin expression in immunocompetent and immunodeficient germ-free and Candida albicans-monoassociated mice. J. Infect. Dis. 190:1327-1334. [DOI] [PubMed] [Google Scholar]
- 27.Shao, X., A. Mednick, M. Alvarez, N. van Rooijen, A. Casadevall, and D. L. Goldman. 2005. An innate immune system cell is a major determinant of species-related susceptibility differences to fungal pneumonia. J. Immunol. 175:3244-3251. [DOI] [PubMed] [Google Scholar]
- 28.Shea, J., T. B. Kechichian, C. Luberto, and M. Del Poeta. 2006. The cryptococcal enzyme inositol phosphosphingolipid-phospholipase C (Isc1) confers resistance to the antifungal effects of macrophages and promotes fungal dissemination to the central nervous system. Infect. Immun. 74:5977-5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thepen, T., N. Van Rooijen, and G. Kraal. 1989. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J. Exp. Med. 170:499-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tucker, S. C., and A. Casadevall. 2002. Replication of Cryptococcus neoformans in macrophages is accompanied by phagosomal permeabilization and accumulation of vesicles containing polysaccharide in the cytoplasm. Proc. Natl. Acad. Sci. USA 99:3165-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Rooijen, N., and A. Sanders. 1994. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods 174:83-93. [DOI] [PubMed] [Google Scholar]
- 32.Wang, B., C. Biron, J. She, K. Higgins, M. J. Sunshine, E. Lacy, N. Lonberg, and C. Terhorst. 1994. A block in both early T lymphocyte and natural killer cell development in transgenic mice with high-copy numbers of the human CD3E gene. Proc. Natl. Acad. Sci. USA 91:9402-9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, B., C. Levelt, M. Salio, D. Zheng, J. Sancho, C. P. Liu, J. She, M. Huang, K. Higgins, M. J. Sunshine, et al. 1995. Over-expression of CD3 epsilon transgenes blocks T lymphocyte development. Int. Immunol. 7:435-448. [DOI] [PubMed] [Google Scholar]
- 34.Wang, B., S. J. Simpson, G. A. Hollander, and C. Terhorst. 1997. Development and function of T lymphocytes and natural killer cells after bone marrow transplantation of severely immunodeficient mice. Immunol. Rev. 157:53-60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.