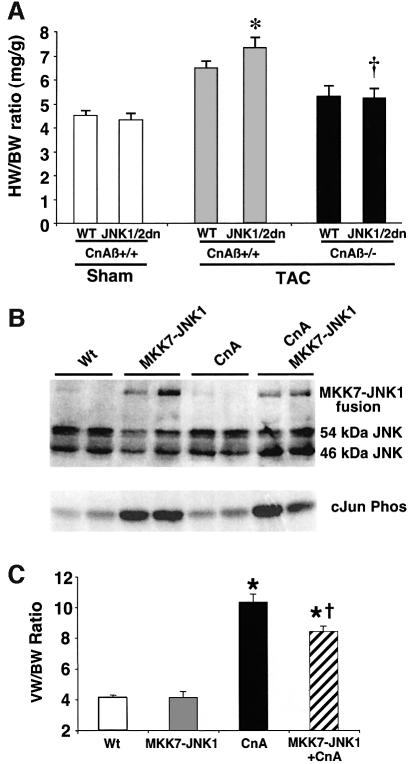
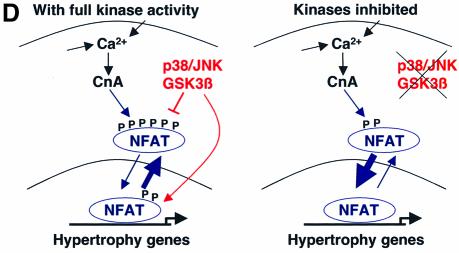
Fig. 7. Calcineurin–NFAT signaling is interconnected with JNK signaling in vivo. (A) The dnJNK1/2 transgene was crossed with calcineurin Aβ gene-targeted mice to generate null mice (CnAβ–/–) or wild-type littermate controls (CnAβ+/+) for gravimetric analysis 1 week after TAC or sham operation. (N = 4–9 mice in each group; *P < 0.05 versus wild-type mice subjected to TAC; †P < 0.05 versus JNK1/2dn subjected to TAC). (B) Western for JNK1/2 protein from the hearts of MKK7–JNK1 fusion transgenic mice (upper band), wild-type mice, activated calcineurin transgenic mice or mice containing both the activated calcineurin and MKK7–JNK1 transgenes. The lower panel shows cJun kinase activity. (C) Analysis of wet ventricle-to-body weight ratios VW/BW from MKK7–JNK1 transgenic mice crossed with activated calcineurin transgenic mice (N = 4 mice in each group; *P < 0.05 versus WT; †P < 0.05 versus CnA transgenic. (D) Model whereby NFAT nuclear translocation and occupancy are controlled by the interplay between calcineurin-mediated dephosphorylation and kinase-mediated phosphorylation (left). Inhibition of a key NFAT kinase, such as JNK, shifts the equilibrium so that the same activation signal by calcineurin now results in greater NFAT translocation and nuclear occupancy.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.