Abstract
1 Intracellular recordings were made from cells of the sinoatrial (S-A) node region and from atrial muscle fibres of rabbit hearts. The effects of sodium salicylate and 5-bromo salicylate on various parameters of the membrane action potential were studied.
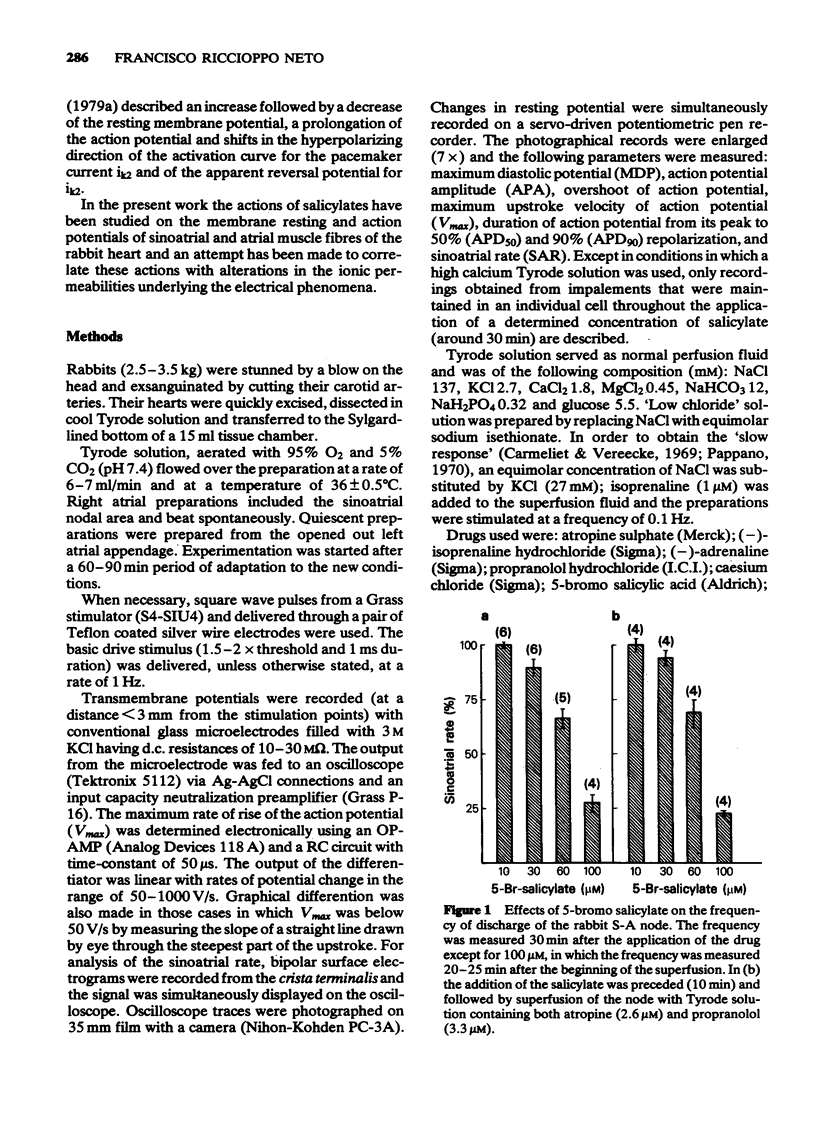
2 5-Bromo salicylate (30-100 μM) and sodium salicylate (300-500 μM) caused a dose-dependent decrease in the frequency of discharge of the SA node cells. Applications of atropine (2.6 μM) with propranolol (3.3 μM) did not affect the negative chronotropic effect, whereas adrenaline (5 μM) reversed it.
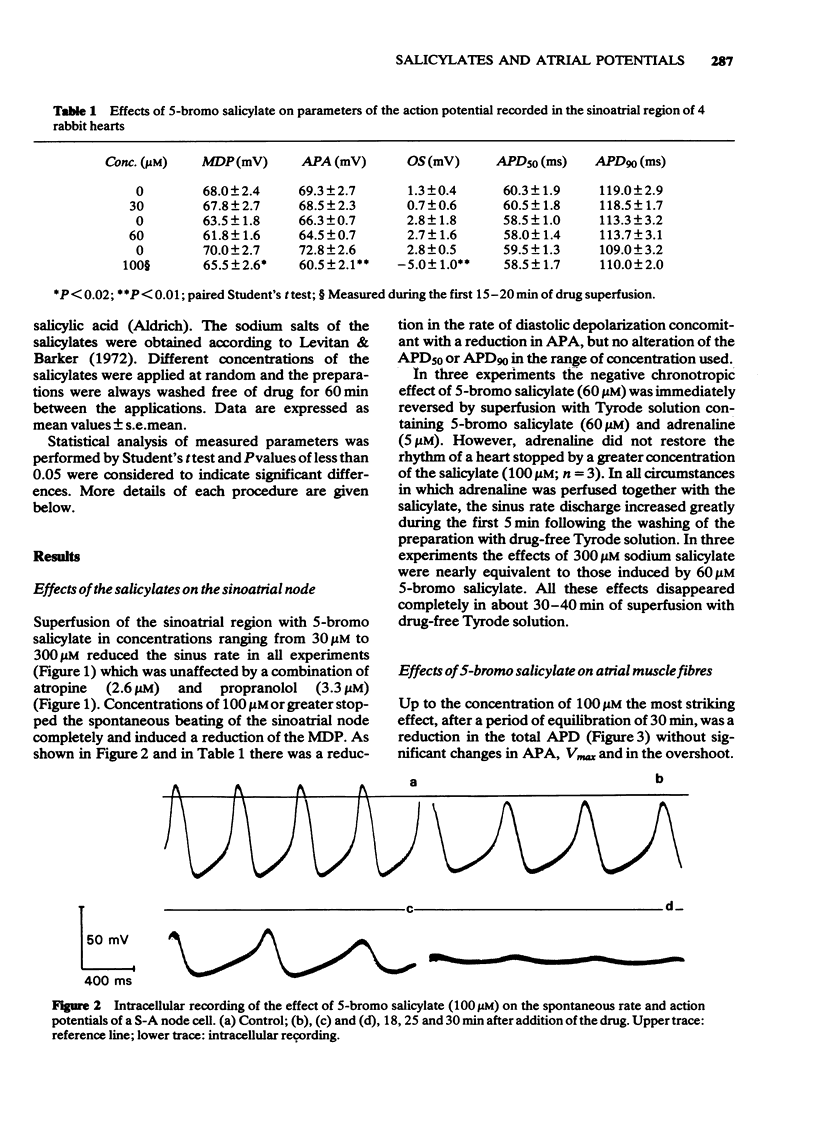
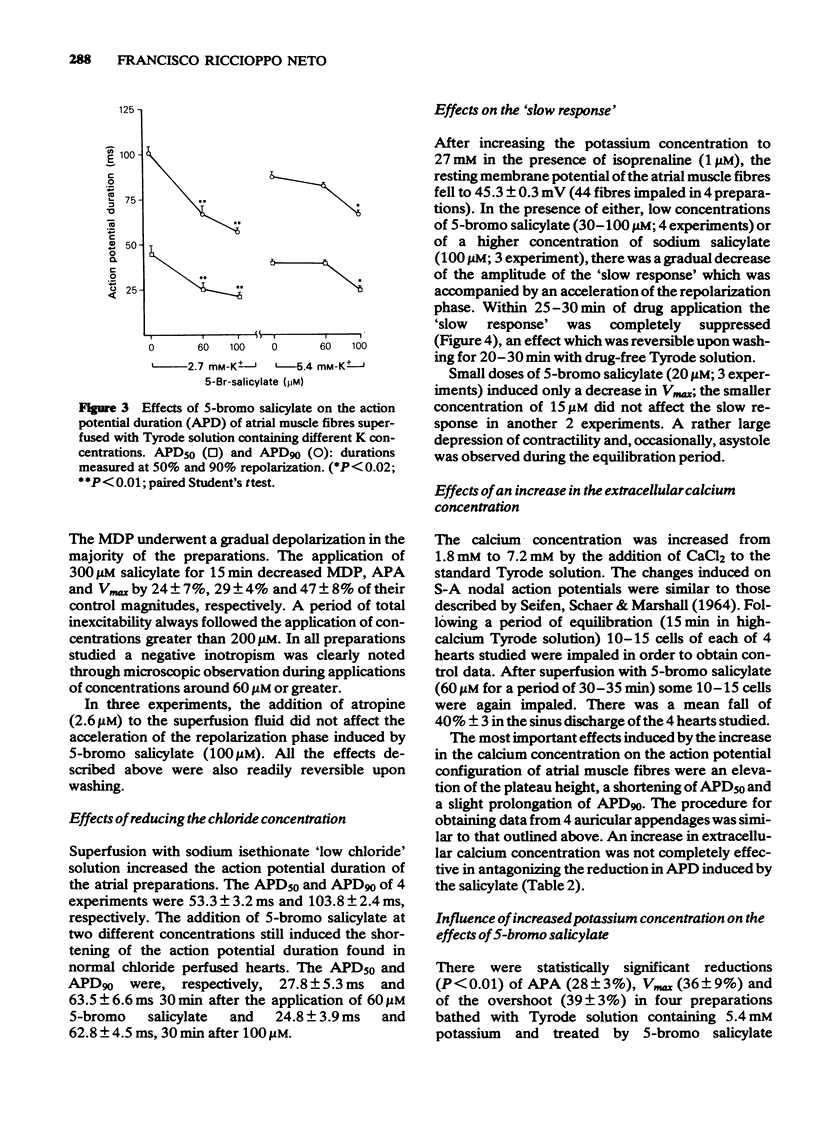
3 Depolarization and shortening of the action potential duration were found in atrial muscle fibres after the application of 5-bromo salicylate (60-100 μM). The reduction of the action potential duration (APD) was not affected by atropine (2.6 μM).
4 Higher concentrations of 5-bromo salicylate (> 100 μM) also caused a dose-dependent reduction in the action potential amplitude (APA), in the overshoot (OS) of the action potential and in the maximum rate of rise of the action potential (Vmax). All these effects were completely reversed on washing.
5 Substitution of the NaCl of the bathing Tyrode solution by an equimolar concentration of Na isethionate did not affect the plateau depression induced by the salicylates in atrial muscle fibres.
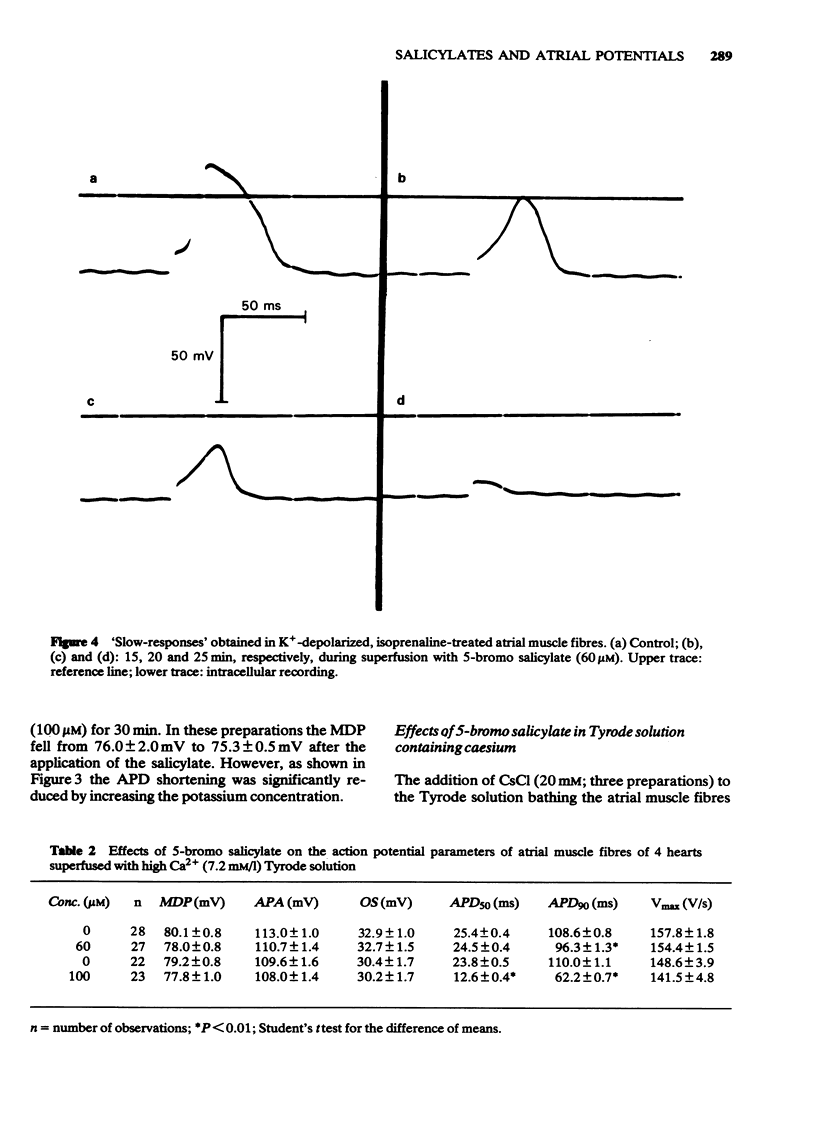
6 After increasing the K concentration to 27 mM in the presence of isoprenaline (1 μM), `slow responses' were obtained upon stimulation. 5-Bromo salicylate (20-60 μM) and sodium salicylate (100 μM) decreased reversibly the amplitude and the rate of rise of the `slow response'.
7 A four fold increase in Ca concentration of the standard Tyrode solution did not antagonize the plateau depression of atrial muscle fibres or the negative chronotropism induced by salicylates.
8 Addition of CsCl (10 mM) to the Tyrode solution did not affect the shortening of the APD induced by the salicylates in atrial muscle fibres.
9 When the K concentration in the Tyrode solution was increased from 2.7 mM to 5.4 mM, the effects of 5-bromo salicylate on the APA, OS and Vmax were potentiated. However, a significant reduction in the shortening of the APD produced by the salicylate was observed.
10 It is suggested that the salicylates possibly depress the slow inward current in both S-A node cells and atrial muscle fibres of the rabbit heart. In atrial muscle fibres, a concomitant increase in the outward potassium current is probably involved.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attwell D., Bergman C., Ojeda C. The action of salicylate ions on the frog node of Ranvier. J Physiol. 1979 Oct;295:69–81. doi: 10.1113/jphysiol.1979.sp012955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Levitan H. Salicylate: effect on membrane permeability of molluscan neurons. Science. 1971 Jun 18;172(3989):1245–1247. doi: 10.1126/science.172.3989.1245. [DOI] [PubMed] [Google Scholar]
- Bassingthwaighte J. B., Fry C. H., McGuigan J. A. Relationship between internal calcium and outward current in mammalian ventricular muscle; a mechanism for the control of the action potential duration? J Physiol. 1976 Oct;262(1):15–37. doi: 10.1113/jphysiol.1976.sp011583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H., Difrancesco D. Voltage-clamp investigations of membrane currents underlying pace-maker activity in rabbit sino-atrial node. J Physiol. 1980 Nov;308:331–351. doi: 10.1113/jphysiol.1980.sp013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet E., Vereecke J. Adrenaline and the plateau phase of the cardiac action potential. Importance of Ca++, Na+ and K+ conductance. Pflugers Arch. 1969;313(4):300–315. doi: 10.1007/BF00593955. [DOI] [PubMed] [Google Scholar]
- Cohen I., Noble D., Ohba M., Ojeda C. Action of salicylate ions on the electrical properties of sheep cardiac Purkinje fibres. J Physiol. 1979 Dec;297(0):163–185. doi: 10.1113/jphysiol.1979.sp013033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch H. G. Characteristics of active Na transport in intact cardiac cells. Am J Physiol. 1979 Feb;236(2):H189–H199. doi: 10.1152/ajpheart.1979.236.2.H189. [DOI] [PubMed] [Google Scholar]
- Irisawa H. Comparative physiology of the cardiac pacemaker mechanism. Physiol Rev. 1978 Apr;58(2):461–498. doi: 10.1152/physrev.1978.58.2.461. [DOI] [PubMed] [Google Scholar]
- Isenberg G. Cardiac Purkinje fibers: cesium as a tool to block inward rectifying potassium currents. Pflugers Arch. 1976 Sep 30;365(2-3):99–106. doi: 10.1007/BF01067006. [DOI] [PubMed] [Google Scholar]
- KLEINFELD M., MAGIN J., STEIN E. Effect of 2,4 dinitrophenol on electrical and mechanical activities of isolated heart. Am J Physiol. 1961 Sep;201:467–470. doi: 10.1152/ajplegacy.1961.201.3.467. [DOI] [PubMed] [Google Scholar]
- Katzung B. G., Morgenstern J. A. Effects of extracellular potassium on ventricular automaticity and evidence for a pacemaker current in mammalian ventricular myocardium. Circ Res. 1977 Jan;40(1):105–111. doi: 10.1161/01.res.40.1.105. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Noma A., Irisawa H. Electrogenic sodium pump in rabbit atrio-ventricular node cell. Pflugers Arch. 1981 Oct;391(4):261–266. doi: 10.1007/BF00581504. [DOI] [PubMed] [Google Scholar]
- Levitan H., Barker J. L. Membrane permeability: cation selectivity reversibly altered by salicylate. Science. 1972 Oct 6;178(4056):63–64. doi: 10.1126/science.178.4056.63. [DOI] [PubMed] [Google Scholar]
- MACFARLANE W. V. The plateau of the action potential of the frog ventricle. Circ Res. 1960 Jan;8:47–56. doi: 10.1161/01.res.8.1.47. [DOI] [PubMed] [Google Scholar]
- McDonald T. F., MacLeod D. P. DNP-induced dissipation of ATP in anoxic ventricular muscle. J Physiol. 1973 Mar;229(3):583–599. doi: 10.1113/jphysiol.1973.sp010155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. F., Trautwein W. The potassium current underlying delayed rectification in cat ventricular muscle. J Physiol. 1978 Jan;274:217–246. doi: 10.1113/jphysiol.1978.sp012144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. Salicylates and phospholipid bilayer membranes. Nature. 1973 May 25;243(5404):234–236. doi: 10.1038/243234a0. [DOI] [PubMed] [Google Scholar]
- Neto F. R., Narahashi T. Ionic mechanism of the salicylate block of nerve conduction. J Pharmacol Exp Ther. 1976 Nov;199(2):454–463. [PubMed] [Google Scholar]
- Noma A., Kotake H., Irisawa H. Slow inward current and its role mediating the chronotropic effect of epinephrine in the rabbit sinoatrial node. Pflugers Arch. 1980 Oct;388(1):1–9. doi: 10.1007/BF00582621. [DOI] [PubMed] [Google Scholar]
- Pappano A. J. Calcium-dependent action potentials produced by catecholamines in guinea pig atrial muscle fibers depolarized by potassium. Circ Res. 1970 Sep;27(3):379–390. doi: 10.1161/01.res.27.3.379. [DOI] [PubMed] [Google Scholar]
- Riccioppo Neto F. Further studies on the actions of salicylates on nerve membranes. Eur J Pharmacol. 1980 Nov 21;68(2):155–162. doi: 10.1016/0014-2999(80)90316-7. [DOI] [PubMed] [Google Scholar]
- SEIFEN E., SCHAER H., MARSHALL J. M. EFFECT OF CALCIUM ON THE MEMBRANE POTENTIALS OF SINGLE PACEMAKER FIBRES AND ATRIAL FIBRES IN ISOLATED RABBITS ATRIA. Nature. 1964 Jun 20;202:1223–1224. doi: 10.1038/2021223a0. [DOI] [PubMed] [Google Scholar]
- Singh B. N., Williams E. M. Effect of altering potassium concentration on the action of lidocaine and diphenylhydantoin on rabbit atrial and ventricular muscle. Circ Res. 1971 Sep;29(3):286–295. doi: 10.1161/01.res.29.3.286. [DOI] [PubMed] [Google Scholar]
- Wit A. L., Cranefield P. F. Effect of verapamil on the sinoatrial and atrioventricular nodes of the rabbit and the mechanism by which it arrests reentrant atrioventricular nodal tachycardia. Circ Res. 1974 Sep;35(3):413–425. doi: 10.1161/01.res.35.3.413. [DOI] [PubMed] [Google Scholar]
- Zipes D. P., Fischer J. C. Effects of agents which inhibit the slow channel on sinus node automaticity and atrioventricular conduction in the dog. Circ Res. 1974 Feb;34(2):184–192. doi: 10.1161/01.res.34.2.184. [DOI] [PubMed] [Google Scholar]
- de Carvalho A. P., Hoffman B. F., de Carvalho M. P. Two components of the cardiac action potential. I. Voltage-time course and the effect of acetylcholine on atrial and nodal cells of the rabbit heart. J Gen Physiol. 1969 Nov;54(5):607–635. doi: 10.1085/jgp.54.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]


