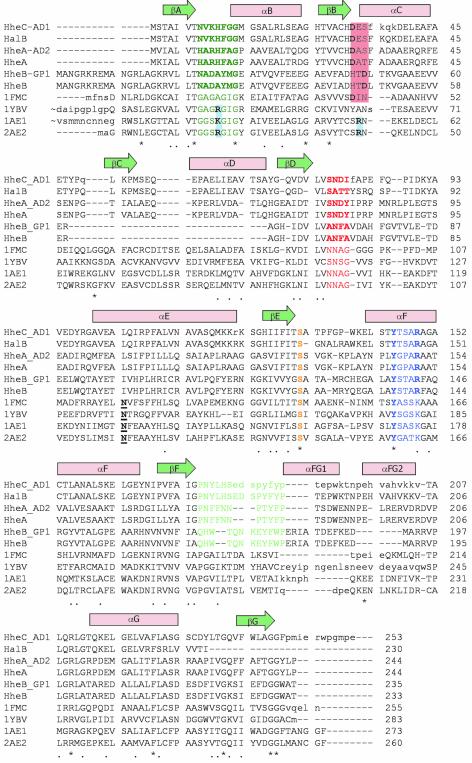
Fig. 4. Sequence alignment of the haloalcohol dehalogenases HalB (Agrobacterium tumefaciens, unpublished, DDBJ/EMBL/GenBank accession No. AAD34609), HheAAD2 (Arthrobacter sp. AD2; van Hylckama-Vlieg et al., 2002), HheA (Corynebacterium sp. N-1074; Yu et al., 1994), HheBGP1 (Mycobacterium sp. GP1; Poelarends et al., 1999) and HheB (Corynebacterium sp. strain N-1074; Yu et al., 1994), combined with a 3D-structure-based alignment of HheC (A.radiobacter AD1; van Hylckama-Vlieg et al., 2001), and the four homologous SDR structures with the highest structural similarity to HheC [7α-hydroxysteroid dehydrogenase from E.coli, PDB accession code 1FMC (Tanaka et al., 1996); trihydroxynaphtalene reductase from M.grisea, PDB accession code 1YBV (Andersson et al., 1996); and tropinone reductases I and II from D.stramonium, PDB accession codes 1AE1 and 2AE1, respectively (Nakajima et al., 1998; Yamashita et al., 1999)]. The colored amino acids correspond to the sequence motifs in Figure 2C and share the same color coding. The underlined bold amino acids correspond to the conserved asparagine in SDR enzymes (Asn118 in 7α-HSD) (Filling et al., 2002). The red- and blue-colored boxes, respectively, indicate the possible positions of acidic and basic residues that define the NADH or NADPH cofactor specificity in the SDR family (Persson et al., 2003).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.