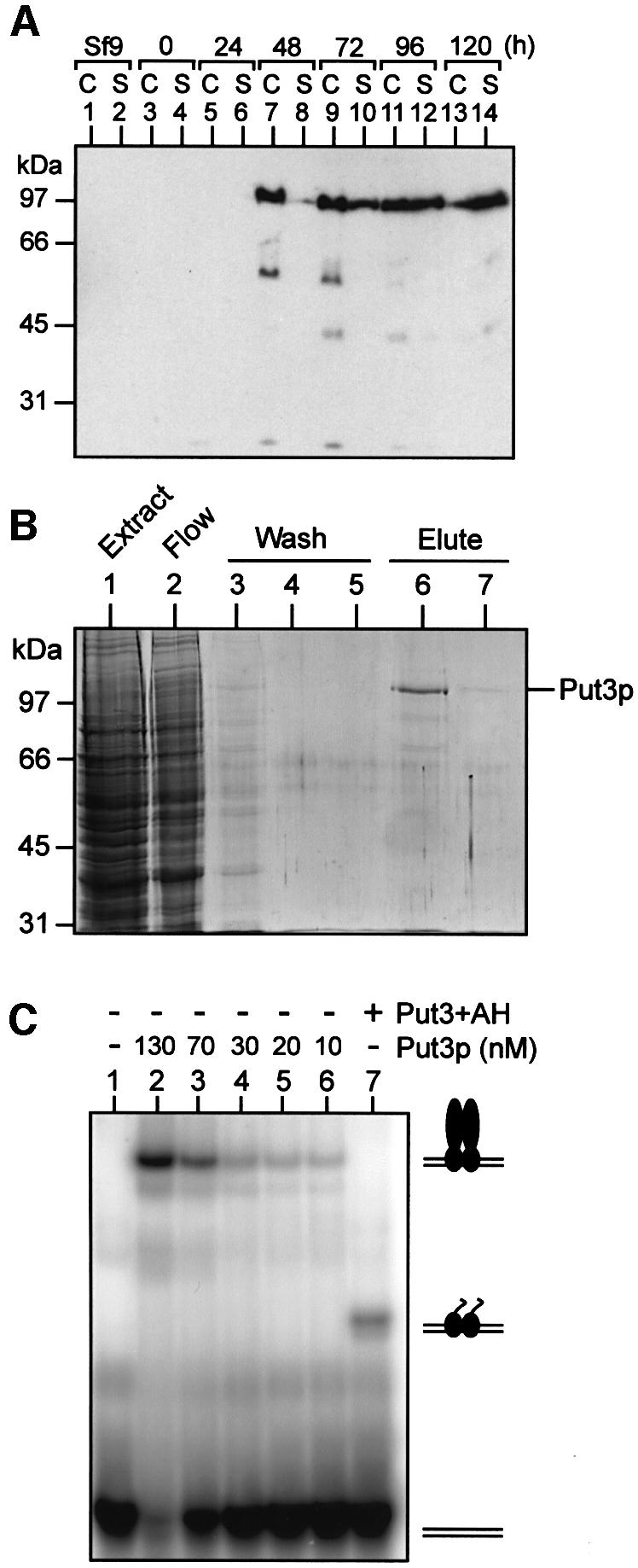
Fig. 1. Over-production and purification of functional Put3p. (A) Time course of Put3p expression in baculovirus-infected insect cells. Cultures of High Five insect cells (50% confluent) were infected at time 0 with recombinant baculovirus containing the PUT3 gene under the control of the polyhedrin promoter. Samples of the cells (C) or the culture supernatant (S) were taken at the times indicated and analysed by western blotting. (B) Purification of Put3p. Insect cells infected with recombinant PUT3 baculovirus were harvested 3 days post-infection. Cell extracts were prepared and soluble protein applied to a Ni2+-NTA agarose column. The column flow-through is shown in lane 2. The column was washed with buffer containing 30 mM imidazole (lanes 3–5) and then Put3p was eluted with buffer containing 250 mM imidazole (lanes 6 and 7). Samples of each fraction were run on an SDS–polyacrylamide gel that was subsequently stained with Coomassie Blue. The sizes of molecular weight standards (in kDa) are indicated. (C) DNA-binding properties of purified Put3p. Electrophoretic mobility shift assays were performed as described in Materials and methods. Reactions contained 32P-labelled DNA comprising a single Put3p-binding site and, as indicated, either purified full-length Put3p, at the concentrations shown, or Put3+AH (500 nM). The positions of the free DNA and the protein–DNA complexes are indicated.
