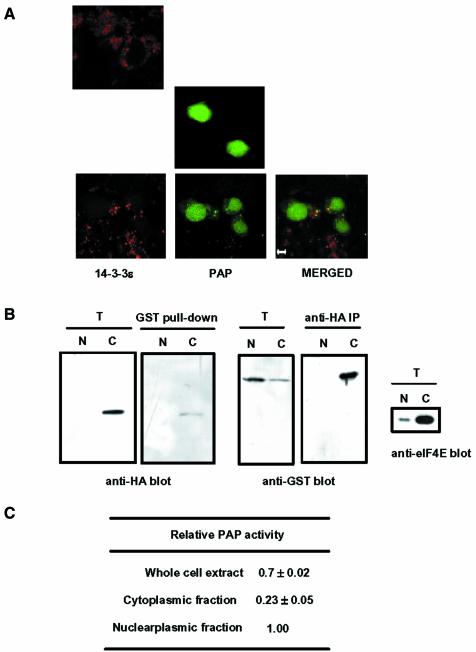
Fig. 8. Subcellular localization of poly(A) polymerase (PAP) and 14-3-3ε. (A) NIH 3T3 cells were transfected with the GFP–PAP construct, the HA-14-3-3ε construct or both together. To visualize the HA-14-3-3ε localization, we incubated the transfected cells with an anti-HA antibody followed by a rhodamine-conjugated anti-rat antibody (Sigma). GFP–PAP and HA-14-3-3ε were visualized with a confocal microscope for green and red fluorescence, respectively. Cells transfected with HA-14-3-3ε alone (top), GFP–PAP alone (middle) or with both GFP–PAP and HA-14-3-3ε (bottom) demonstrate typical localization of the recombinant proteins. The bottom panel also shows a merged image of the two fluorescence emissions of the same image. Scale bar: 5 µm. (B) HeLa cells were transfected the GST–PAP and HA-14-3-3ε constructs. Nucleoplasmic and cytoplasmic fractions were isolated and each fraction was subjected to coimmunprecipitation analysis, as in Figure 3A. The same amount in the cell number was used for each fraction. The preparation of the nucleoplasmic and cytoplasmic fractions was ensured by immunoblotting with anti-eIF2E antibody. T, total lysates (2% input); N, nucleoplasmic fraction; C, cytoplasmic fraction. (C) GST–PAP of the nucleoplasmic and cytoplasmic fractions was purified by immobilization on glutathione–Sepharose beads. Non-specific polyadenylation activity of GST–PAP immobilized on the beads was determined by the trichloroacetic acid precipitation assay, as in Table II. The relative PAP activity represents the amount of [α-32P]ATP incorporated by GST–PAP from the whole cell extract or the cytoplasmic fraction relative to that by GST–PAP from the nucleoplasmic fraction. The equivalent of GST–PAP in the cell number was used for each fraction.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.