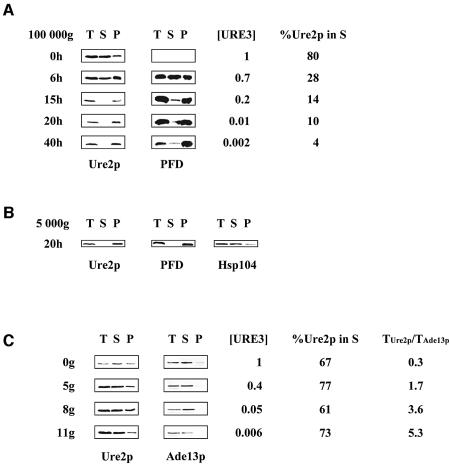
Fig. 3. The kinetics of Ure2p solubility following PFD overproduction and GdnHCl treatment. (A) Overproduction of the PFD leads to Ure2p aggregation. CC34 [URE3] cells transformed with pYe2T-Nter, for overproduction of the PFD, were cultured as described in Figure 1B. At various times after induction, we determined [URE3] frequency, the subcellular distribution of Ure2p and PFD, and the proportion of Ure2p in the 100 000 g supernatant fraction (%Ure2p in S), as described in Materials and methods, using an affinity-purified polyclonal anti-Ure2p antibody. T, total protein extract; S, soluble fraction; P, pellet fraction. (B) Ure2p and the PFD are found in the 5 000 g pellet. CC34 [URE3] cells transformed with pYe2T-Nter, for overproduction of the PFD, were cultured for 20 h in galactose medium (as described in Figure 1B). The total protein extract was centrifuged at 5 000 g for 30 min (see Materials and methods). The membrane was probed using an affinity-purified polyclonal anti-Ure2p antibody and a polyclonal anti-Hsp104 antibody. T, total protein extract; S, soluble fraction; P, pellet fraction. (C) GdnHCl does not affect Ure2p solubility. CC34 [URE3] cells were grown in YPD supplemented with 5 mM GdnHCl. After 0, 5, 8 and 11 generations in the presence of 5 mM GdnHCl (0g, 5g, 8g and 11g), we determined the subcellular distribution of Ure2p, [URE3] frequency and the proportion of Ure2p in the supernatant fraction as described in (A). Western blotting with a polyclonal anti-Ade13p antibody was used to determine Ade13p levels. To assess Ure2p levels in the total protein extract from the cells analysed, we calculated the ratio of Ure2p levels in total protein extract to Ade13p levels in total protein extract (TUre2p/TAde13p). %Ure2p in S; T, S, and P are as defined for (A).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.