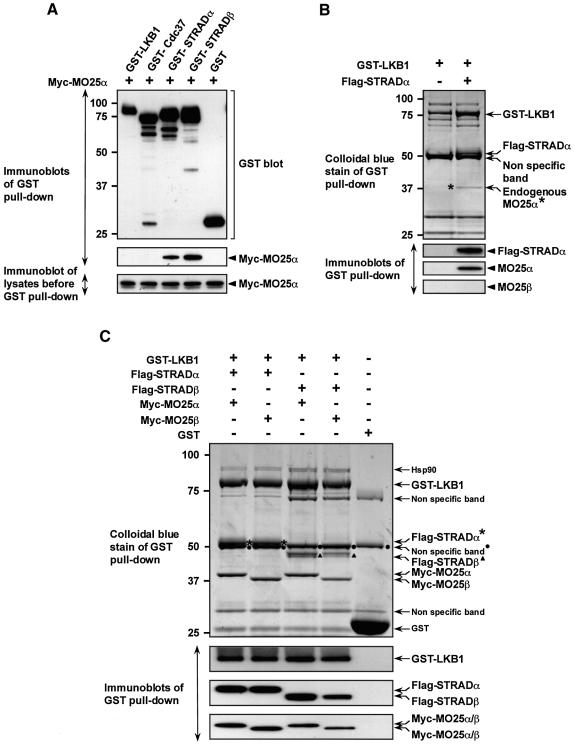
Fig. 4. MO25α is associated with LKB1 through STRAD. (A) 293 cells were transfected with plasmids encoding for the expression of the indicated GST fusion proteins together with Myc-MO25α. Thirty-six hours post-transfection, the GST-tagged proteins were affinity purified from the cell lysates using glutathione–Sepharose as described in Materials and methods. Similar amounts of the purified GST fusion proteins were subjected to SDS–PAGE and immunoblotted with anti-Myc antibody to detect co-purified Myc-MO25α, or with anti-GST antibody to ensure that comparable amounts of the GST-tagged proteins were present in each lane (upper and middle panels). Total cell lysates (5 µg) prior to affinity purification were also subjected to immunoblotting with anti-Myc antibody to ensure that Myc-MO25α was expressed at similar levels in each co-transfection (lower panel). (B) N-terminal GST-tagged LKB1 was expressed in 293 cells in the presence or absence of Flag-STRADα and purified as above. The purified proteins were subjected to SDS–PAGE and visualized by colloidal Coomassie Blue staining (upper panel). The faint protein band indicated, corresponding to an endogenous protein of 40-kDa (marked *), was identified by tryptic peptide mass-spectral fingerprint as endogenously expressed MO25α (with 38% sequence coverage). The purified proteins were also immunoblotted with the indicated antibodies (lower panels). (C) As above except that GST–LKB1 was expressed in 293 cells together with the indicated isoforms of Flag-STRAD and Myc-MO25 and the proteins visualized by colloidal Coomassie Blue staining were also immunoblotted with the indicated antibodies. For all panels, similar results were obtained in at least three separate experiments.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.