Abstract
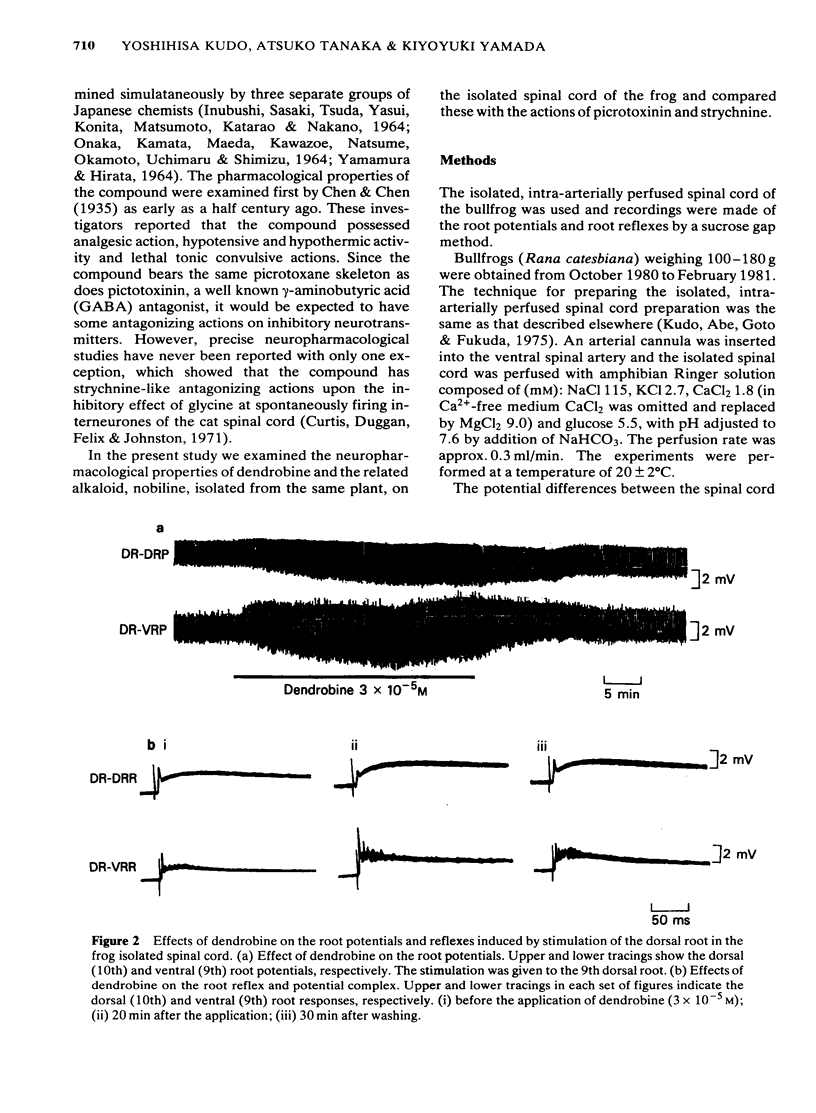
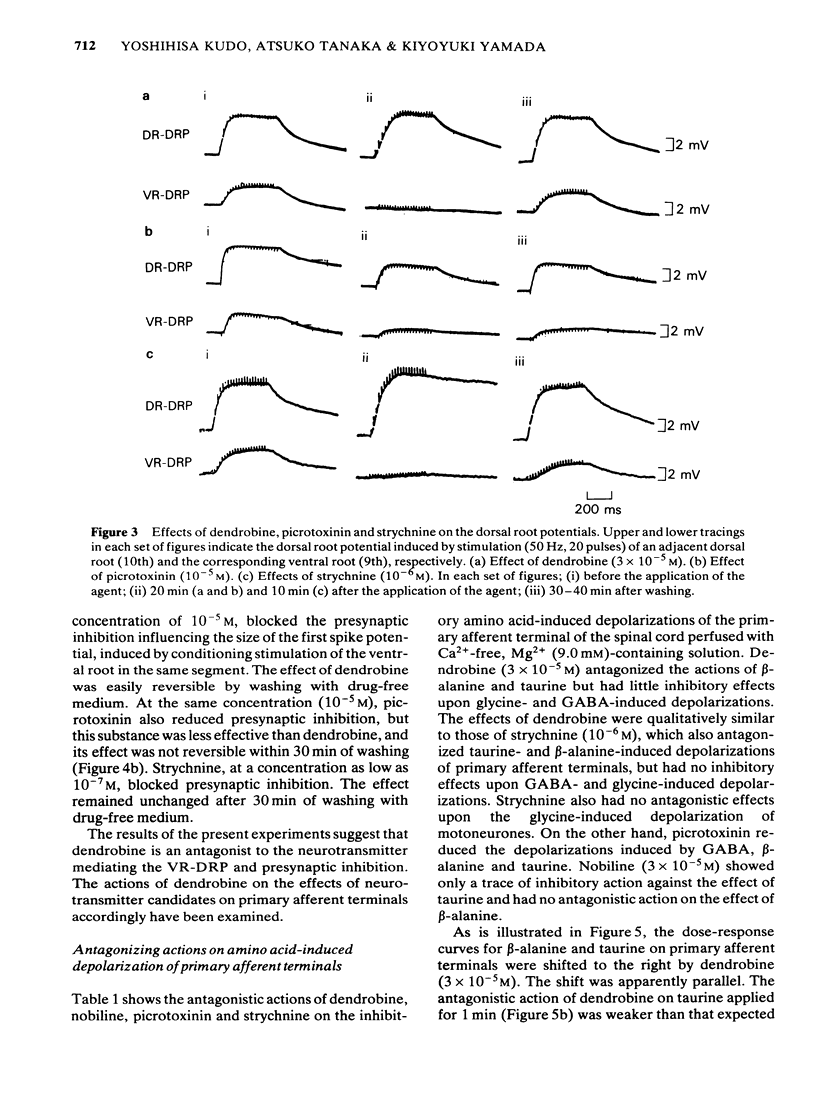
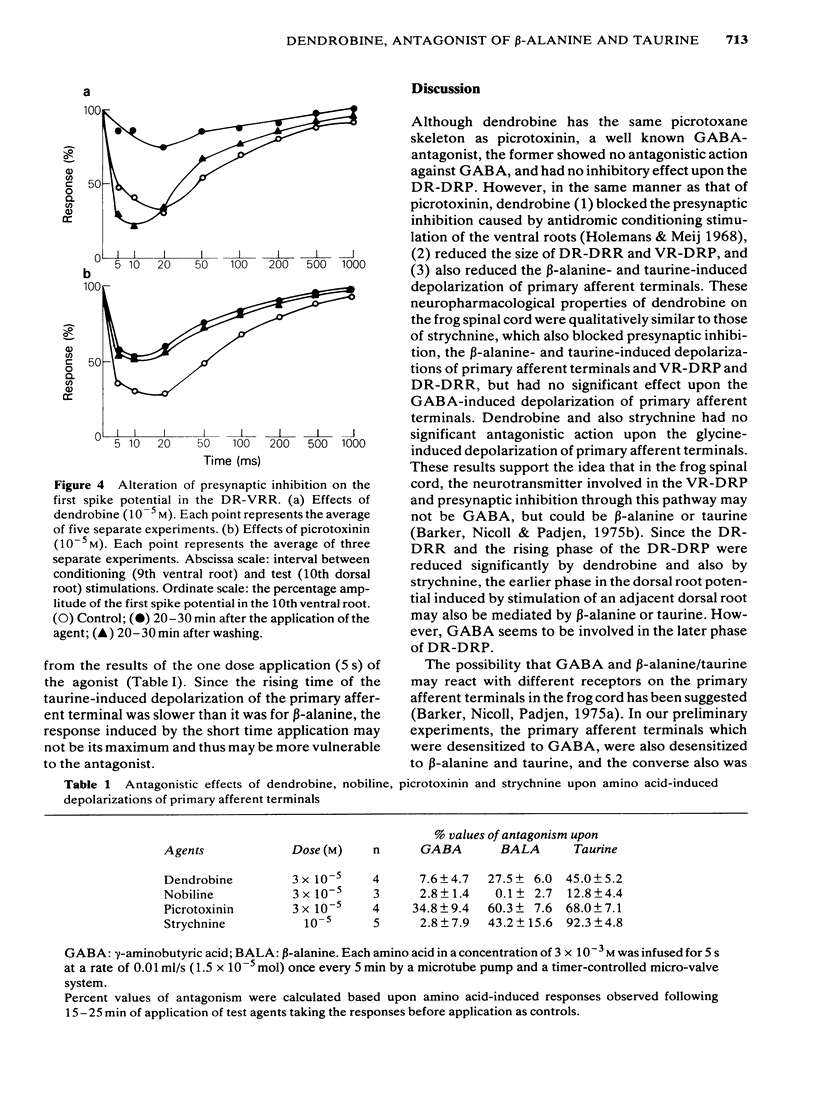
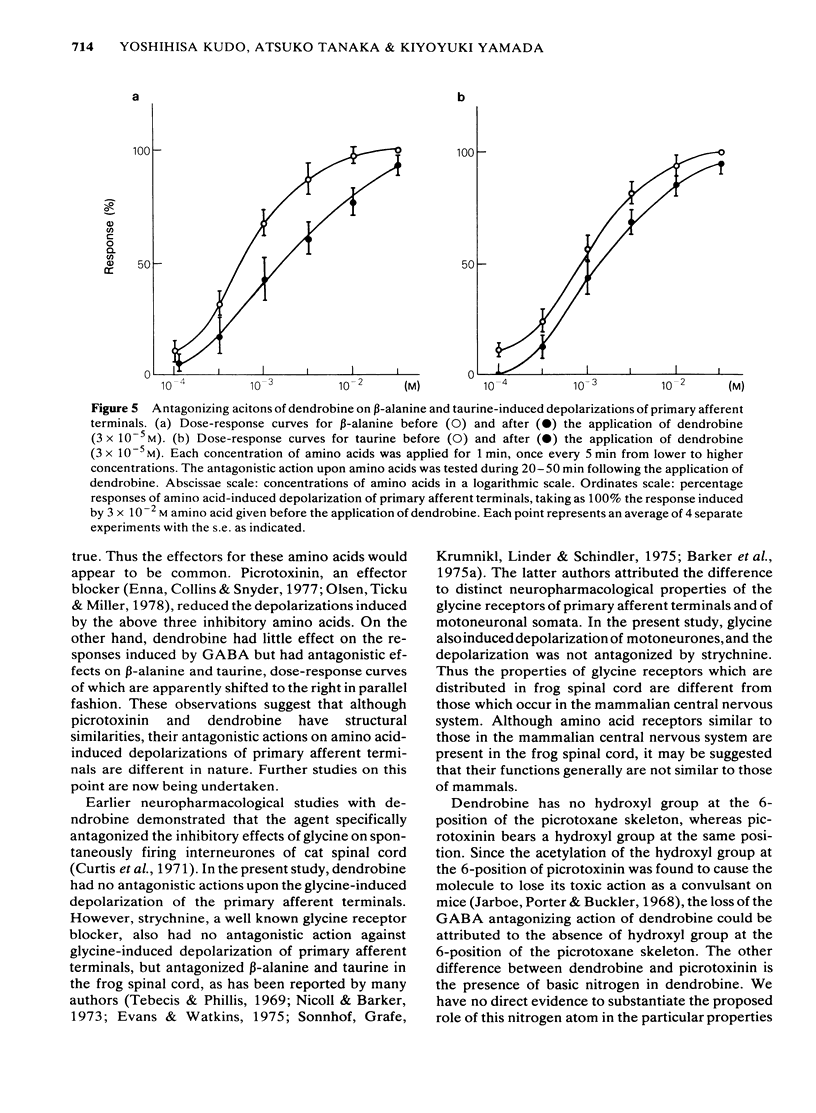
1 The effects of dendrobine and nobiline, alkaloids isolated from Dendrobium nobile, on the electrical activity and on amino acid-induced depolarizations of primary afferent terminals were tested on the frog isolated spinal cord and were compared with those of picrotoxinin and strychnine. 2 Dendrobine (3 X 10(-5) M) caused a slight hyperpolarization in both dorsal and ventral roots and this hyperpolarization was accompanied by the augmentation of the dorsal root potential (DR-DRP) and the ventral root potential and reflex (DR-VRP and DR-VRR). The amplitude of the dorsal root reflex (DR-DRR) however, was reduced significantly. Nobiline (3 X 10(-5) M) had no significant effect on either the root potentials or the reflexes. 3 Dendrobine (3 X 10(-5) M) reduced the dorsal root potential induced by repetitive antidromic stimulation of ventral root (VR-DRP) as well as diminishing the maximum rate of rise of the dorsal root potential induced by the stimulation of adjacent dorsal roots (DR-DRP), during which time the amplitude of the DR-DRP was seen to be augmented. 4 Dendrobine (3 X 10(-5) M) reduced the beta-alanine- and taurine-induced depolarizations of primary afferent terminals, while having little effect upon GABA- and glycine-induced depolarizations. 5 Dendrobine (10(-5) M) reversibly blocked the presynaptic inhibition caused by antidromic conditioning stimulation of the ventral root. 6 These effects of dendrobine were qualitatively similar to those of strychnine but were somewhat different from those of picrotoxinin, a molecule having the same picrotoxane skeleton. 7 The present results are discussed with reference to the likely neurotransmitters involved in presynaptic inhibition in the frog spinal cord, and with respect to the structure-activity relationship of picrotoxane compounds as amino acid antagonists.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker J. L., Nicoll R. A., Padjen A. Studies on convulsants in the isolated frog spinal cord. I. Antagonism of amino acid responses. J Physiol. 1975 Mar;245(3):521–536. doi: 10.1113/jphysiol.1975.sp010859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Nicoll R. A., Padjen A. Studies on convulsants in the isolated frog spinal cord. II. Effects on root potentials. J Physiol. 1975 Mar;245(3):537–548. doi: 10.1113/jphysiol.1975.sp010860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A. Bicuculline, an antagonist of GABA and synaptic inhibition in the spinal cord of the cat. Brain Res. 1971 Sep 10;32(1):69–96. doi: 10.1016/0006-8993(71)90156-9. [DOI] [PubMed] [Google Scholar]
- Enna S. J., Collins J. F., Snyder S. H. Stereospecificity and structure--activity requirements of GABA receptor binding in rat brain. Brain Res. 1977 Mar 18;124(1):185–190. doi: 10.1016/0006-8993(77)90878-2. [DOI] [PubMed] [Google Scholar]
- Evans R. H., Watkins J. C. Ventral root responses of the hemisected amphibian spinal cord to perfused amino acids in the presence of procaine. Br J Pharmacol. 1975 Dec;55(4):519–526. doi: 10.1111/j.1476-5381.1975.tb07427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarboe C. H., Poerter L. A., Buckler R. T. Structural aspects of picrotoxinin action. J Med Chem. 1968 Jul;11(4):729–731. doi: 10.1021/jm00310a020. [DOI] [PubMed] [Google Scholar]
- Kudo Y., Abe N., Goto S., Fukuda H. The chloride-dependent depression by GABA in the frog spinal cord. Eur J Pharmacol. 1975 Jun-Jul;32(02):251–259. doi: 10.1016/0014-2999(75)90290-3. [DOI] [PubMed] [Google Scholar]
- Kudo Y. The pharmacology of the amphibian spinal cord. Prog Neurobiol. 1978;11(1):1–76. doi: 10.1016/0301-0082(78)90007-2. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Barker J. L. Effect of strychnine on dorsal root potentials and amino acid responses in frog spinal cord. Nat New Biol. 1973 Dec 19;246(155):224–225. doi: 10.1038/newbio246224a0. [DOI] [PubMed] [Google Scholar]
- ONAKA T., KAMATA S., MAEDA T., KAWAZOE Y., NATSUME M., OKAMOTO T., UCHIMARU F., SHIMIZU M. THE STRUCTURE OF DENDROBINE. Chem Pharm Bull (Tokyo) 1964 Apr;12:506–512. doi: 10.1248/cpb.12.506. [DOI] [PubMed] [Google Scholar]
- Olsen R. W., Ticku M. K., Miller T. Dihydropicrotoxinin binding to crayfish muscle sites possibly related to gamma-aminobutyric acid receptor-ionophores. Mol Pharmacol. 1978 May;14(3):381–390. [PubMed] [Google Scholar]
- Sonnhof U., Grafe P., Krumnikl J., Linder M., Schindler L. Inhibitory postsynaptic actions of taurine, GABA and other amino acids on motoneurons of the isolated frog spinal cord. Brain Res. 1975 Dec 19;100(2):327–341. doi: 10.1016/0006-8993(75)90486-2. [DOI] [PubMed] [Google Scholar]
- Tebecis A. K., Phillis J. W. The use of convulsants in studying possible functions of amino acids in the toad spinal cord. Comp Biochem Physiol. 1969 Mar;28(3):1303–1315. doi: 10.1016/0010-406x(69)90568-4. [DOI] [PubMed] [Google Scholar]


