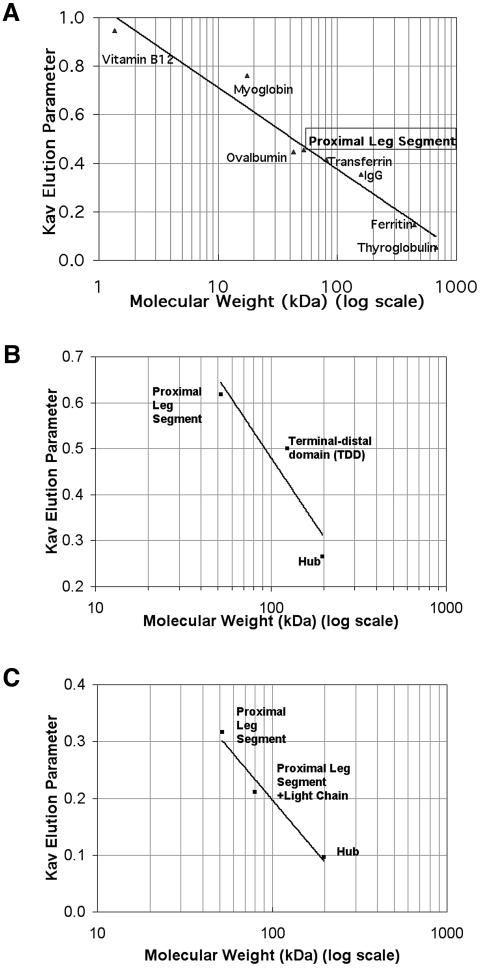
Fig. 2. Leg segments are monomeric by FPLC. Fragments representing the clathrin proximal leg and terminal–distal domain were expressed as polyhistidine-tagged recombinant proteins and analyzed by gel permeation chromatography. Kav versus molecular weight for sample and standards were plotted. Proximal leg segments are monomeric on a calibrated FPLC column. (A) Proximal leg fragment and calibration standards were independently chromatographed on a Superdex 200 HR 10/30 column in Bis–TRIS pH 6.2 and elution times were noted. Proximal leg segment Kav was calculated as 0.454, indicating a size for clathrin proximal leg segment consistent with a monomeric 52 kDa species. (B) Isolated proximal and distal leg segments are monomeric and do not form a complex when combined. Proximal and terminal-distal domains were chromatographed on a Superose 6 prep grade column in MES/TRIS pH 6.7 and elution times were compared with elution of Hub or leg segments at pH 7.3. Kav was calculated. Mixtures of proximal and distal domain segments eluted as two separate populations; no larger peak corresponding to a complex was observed. (C) Proximal leg segments bind light chains normally but do not dimerize in the presence of light chain. Proximal leg segments with and without purified light chains and Hub were chromatographed on a Superose 6 prep grade column in Bis–TRIS pH 6.2 and elution times were noted for calculation of Kav. While the mixture of proximal leg with light chain eluted as a complex, indicating proper folding of the recombinant protein fragments, the Kav of the complex is consistent with each complex containing only one light chain and one proximal leg. Thus the presence of light chain did not induce homodimerization of the proximal leg.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.