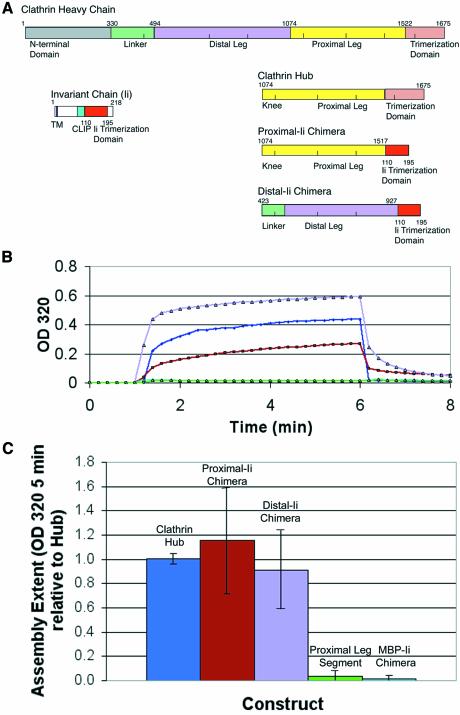
Fig. 5. Chimeric trimerized clathrin proximal or distal leg segments assemble. (A) Chimeric constructs distal-Ii and proximal-Ii were created with clathrin distal or proximal leg segments, respectively, attached to the trimerization domain of the invariant chain protein. The bar diagrams indicate the protein domains of each donor protein from which chimeras were constructed and the domains in the resulting chimeras. Numbers indicate the residues flanking protein domains and the junctions where fragments were recombined. (B) Hub (blue) and purified chimeric proteins proximal-Ii (brown) and distal-Ii (lavender) at pH 7.9 were induced to assemble by a drop in pH 6.7 through the addition of 1/25 volume of 1 M MES pH 6.7, and turbidity (change in OD at 320 nm) was monitored for 5 min. Disassembly was induced by increasing the pH with an addition of 1/25 volume of 1 M TRIS pH 9 and monitoring OD320 for an additional 2 min as turbidity returned to baseline levels, demonstrating reversibility of the assembly process. Monomeric proximal leg segment (green) and trimeric MBP-Ii (cyan) served as controls (overlapping lines near the baseline). (C) The chimeras had a similar overall extent of assembly to purified clathrin Hub in the pH 6.7 turbidity assay. Samples were induced to assemble as above and the extent of assembly (OD320 after 5 min) was recorded. Extent of assembly was normalized to average hub assembly over several preparations and averaged for this plot.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.