Fig. 4. Examination of the rad51 G103E mutant protein for interactions with Rad54.
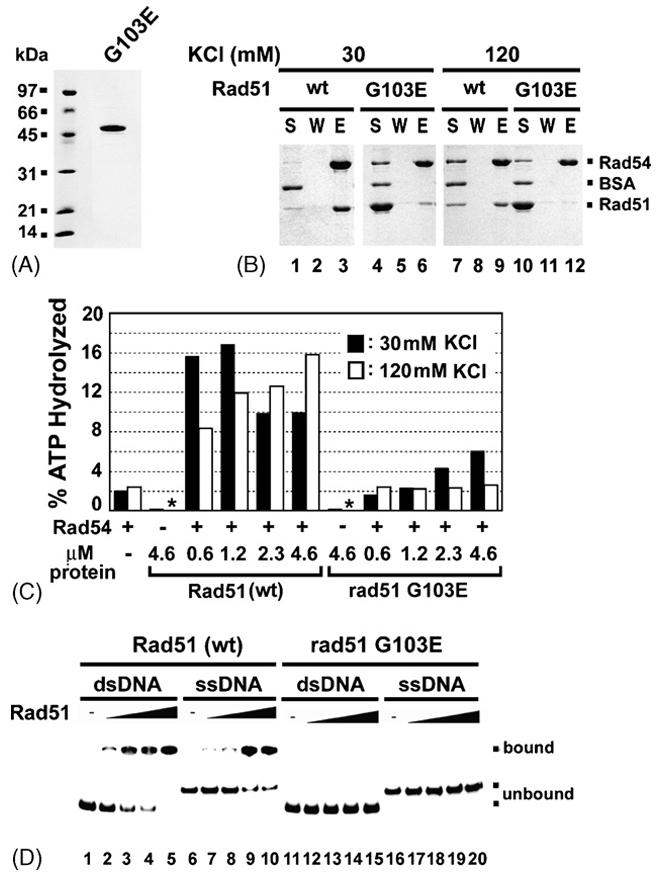
(A) Purified rad51 G103E mutant protein (2 μg) was analyzed by SDS-PAGE.
(B) Affinity pulldown to examine complex formation of rad51 G103E with Rad54, conducted with 30 or 120 mM KCl, as described for Fig. 3A.
(C) The ability of rad51 G103E and Rad51 to stimulate the Rad54 ATPase activity was examined at either 30 mM (black bar) or 120 mM KCl (white bar). ATP hydrolysis was carried out with the indicated amounts of Rad51 or rad51 G103E at 30 °C for 5 min. An asterisk indicates that ATP hydrolysis was not detectable.
(D) Binding of ssDNA and dsDNA by Rad51 (lanes 1-10) or rad51-G103E (lanes 11-20) was examined.
