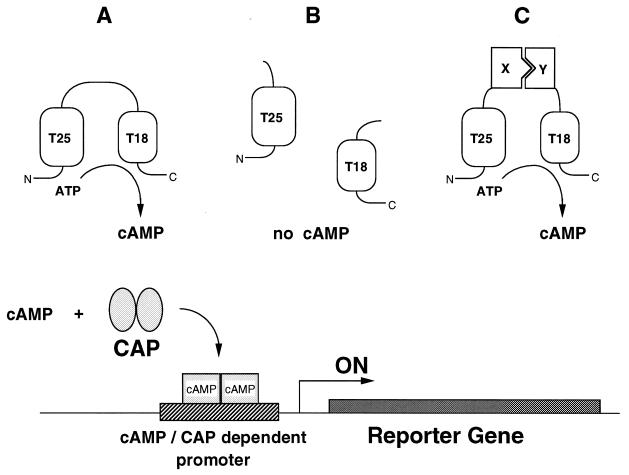
Figure 1.
Principle of an E. coli two-hybrid system based on functional complementation of CyaA fragments. (Upper) Schematic of the basic principle of in vivo complementation between the two fragments of the catalytic domain of B. pertussis adenylate cyclase. The two boxes represent the T25 and T18 fragments corresponding to amino acids 1–224 and 225–399 of the CyaA protein. In A, the full-length catalytic domain (residues 1–399), when expressed in E. coli, exhibits a basal calmodulin-independent activity that results in cAMP synthesis. In B, the two fragments, T25 and T18, when coexpressed as independent polypeptides, are unable to interact and no cAMP synthesis occurs. In C, the two fragments, fused to two interacting proteins, X and Y, are brought into close proximity, resulting in functional complementation followed by cAMP production. (Lower) Schematic of the readout of the complementation. cAMP, synthesized in an E. coli cya strain by the complementing T25 and T18 pairs, binds to the catabolite gene activator protein, CAP. The cAMP/CAP complex then can recognize specific promoters and switch on the transcription of the corresponding genes. These reporter genes can be either natural E. coli genes, such as lacZ or mal genes, or synthetic ones, such as antibiotic-resistance genes fused to a cAMP/CAP-dependent promoter.