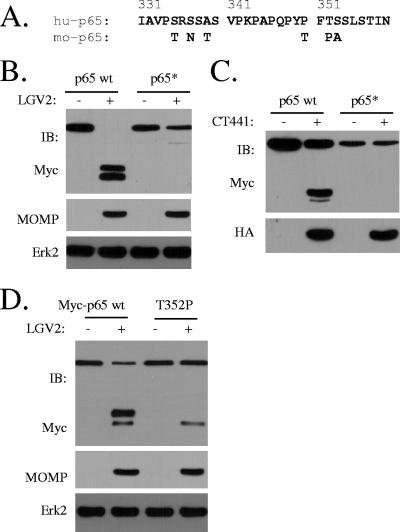
FIG. 5.
Identification of p65 cleavage site by CT441 Tsp. (A) Sequence alignment of the cleavage region of the human (hu-p65) and the cleavage-resistant mouse (mo-p65) p65 proteins. Within the region of cleavage, human and mouse p65 differ by 6 aa residues. (B) A mutant (Myc-p65*) that was prepared by the replacement of the corresponding amino acid residues within the cleavage region of the human p65 protein with the mouse p65 protein was resistant to Chlamydia and CT441 cleavage in 293T cells. Monolayers of 293T cells were transfected for Myc-p65 or Myc-p65* expression. The cells were then infected with LGV2 for 28 h. Protein expression and cleavage of Myc-p65 wt and Myc-p65* were detected with an anti-Myc antibody. MOMP expression was included as a measure of Chlamydia infection. (C) In a parallel experiment, the cells were cotransfected with HA-CT441 and Myc-p65 or Myc-p65*. CT441 expression and p65 protein cleavage were detected with anti-HA and anti-Myc antibodies, respectively. (D) A T352P mutation prevents the primary band production by Chlamydia cleavage. A peptidic bond between a phenylalanine and a nonproline residue resembles a scissile bond of serine proteases. Therefore, a T352P mutant was prepared and tested for Chlamydia cleavage. Note the absence of the slower-migrating protein band in the T352P lane. IB, immunoblot.