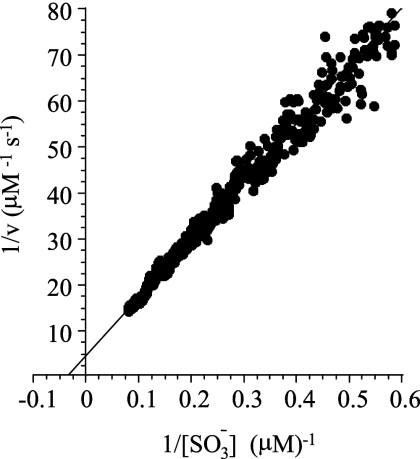
FIG. 5.
Initial rate determination of the Michaelis constants for the SirA-catalyzed reduction of sulfite. Sulfite reduction was monitored continuously (under stringent, anaerobic conditions) via the change in absorbance at 684 nm associated with the oxidation of the electron donor, MV (see Materials and Methods). The assay conditions were as follows: sulfite (13 μM), reduced MV (250 μM), O-acetyl-l-serine (30 μM), SirA (1.3 μM), O-acetyl-l-serine sulfhydrylase (1.2 μM), and HEPES-K+ (50 mM, pH 8.0) (25 ± 3°C). The reaction rate is given in terms of sulfite reduced per unit time (μM/s−1). The reduction of one equivalent of sulfite requires the oxidation of six equivalents of MV. v, initial velocity.