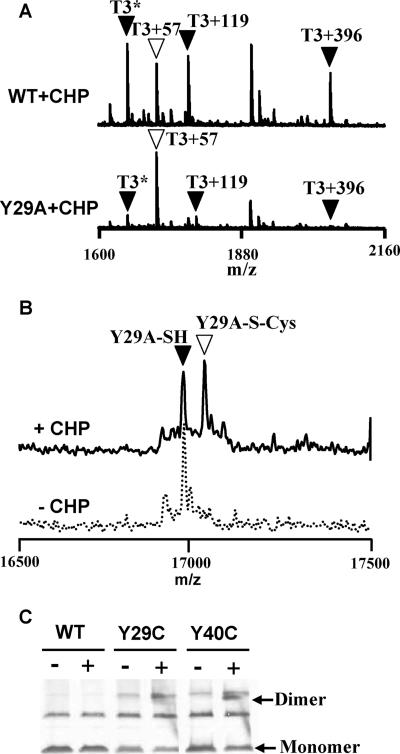
FIG. 3.
Y29A mutant displays reduced oxidation at C15 both in vivo and in vitro. (A) To compare the sensitivity of C15 in the WT and Y29A mutant proteins to oxidation, cells were treated with 100 μM CHP for 1 min, and the epitope (FLAG)-tagged proteins were recovered by immunoprecipitation. MALDI-TOF MS analysis of a tryptic digest of the recovered protein from the WT revealed the expected presence of oxidized C15-containing peptides (filled triangles), including (from left to right) the sulfenamide (T3*), S-cysteinylated peptide (T3+119), and S-thiolated peptide modified with the 398-Da thiol (T3+396), as previously described (10). The open triangle indicates the peak (T3+57) corresponding to reduced OhrR alkylated with IA. In comparison, the Y29A mutant protein was present predominantly in the reduced, and therefore IA-modified, state even after CHP treatment. (B) Y29A mutant protein is only slowly S cysteinylated in vitro. Three hundred nanomolar Y29A OhrR protein was treated with or without 3 μM CHP in the presence of 10 μM cysteine for 10 min. The protein was then TCA precipitated and subjected to ESI-MS. The filled triangle indicates reduced OhrR, whereas the open triangle indicates cysteinylation of OhrR. Note that the Y29A mutant was about half-modified after 10 min of treatment, whereas WT OhrR was similarly modified after only 1 min of treatment and was fully modified by 5 min (see Fig. 4B). (C) Immunoblot analysis of oxidation products of Y29C and Y40C in vivo. Cells were not treated (−) or treated (+) with 100 μM CHP for 1 min before TCA precipitation and analysis by nonreducing SDS-PAGE. A small amount of OhrR dimer was detected for the Y40C mutant (arrow), indicating the presence of a small amount of disulfide cross-linked dimer. Little if any disulfide cross-linked dimer was detected for Y29C. The origin of the upper cross-reactive band, present in the cells containing mutant proteins, was unclear, but this band was not reduced by DTT, indicating that it was unlikely to represent disulfide-linked dimer (data not shown).