Abstract
An assay to measure avidity index (AI) was developed to diagnose incident hepatitis C virus (HCV) infections. The assay demonstrated an AI value statistically significantly lower in primary HCV infections than in chronic infections. When the assay was applied to past resolved infections, the difference in AI values was not as significant as the difference between incident and chronic infections. Lower AI values obtained in past resolved infections may be directly related to lower levels of immunoglobulin G anti-HCV in past resolved infections than in either new infections or chronic infections.
The diagnosis of incident hepatitis C virus (HCV) infections has relied on a combination of epidemiological risk factor assessment and screening for serum alanine aminotransferase activity and HCV antibody seroconversion (2). The identification of incident infection is important in the context of treatment with interferon, because therapy initiated within 3 months after infection prevents the development of chronic infection in nearly all cases (13). Many new infections remain undiagnosed, however, as they are asymptomatic. Furthermore, asymptomatic, primary infections tend to be more associated with lower rates of spontaneous viral clearance than symptomatic infections (10, 12, 13, 15, 17). Therefore, it is clinically important to differentiate incident HCV infections from chronic infections and to identify primary infections that are asymptomatic. Occasional occurrences of acute exacerbation in chronic infection (14) complicate decisions regarding whom to treat.
There are no reliable serological or virological assays applicable to acute-phase serum samples that can distinguish between acute and chronic HCV infection. A number of studies have shown that serum anti-HCV immunoglobulin M (IgM) is not a useful marker for the diagnosis of acute infection (1, 4, 5), unlike the situation with acute hepatitis A and B. The only reliable laboratory means to identify incident infections is to demonstrate seroconversion to anti-HCV (11), which is very difficult to accomplish outside of prospective studies. An alternative approach to the serological diagnosis of primary HCV infections is to measure the avidity of IgG antibody since it is well known that the avidity of antibody increases progressively with time after exposure to an immunogen (7).
Previous studies have indicated the usefulness of determining antibody avidity for the study of acute and chronic HCV infection. Gray and Wreghitt reported on an adaptation of a first-generation enzyme-linked immunosorbent assay (ELISA) for detecting anti-HCV to differentiate nonspecific (low-avidity) antibody binding from specific (high-avidity) binding by antibody generated during long-term infection (8). Ward et al. reported an adaptation of a second-generation anti-HCV ELISA to show that anti-HCV avidity in dialysis patients increases with time after primary infection (16). Furthermore, it was observed in these studies that patients given immunosuppressive therapy did not show a progressive increase in anti-HCV antibody avidity, as was observed for nonimmunosuppressed controls, suggesting that immunosuppression slows the maturation of IgG avidity (8, 16). Another group of investigators (11) reported that anti-HCV avidity was lower in sera taken from patients with resolved infection than in sera from chronically infected patients and that the avidity index (AI) declined in the latter after treatment with interferon. Here, we report on the development of an anti-HCV IgG avidity test using a novel combination of target antigens and its validation when applied to seroconversion panels and samples taken from chronically infected individuals and from those with resolved infection.
Specimens.
Two hundred twenty plasma samples were obtained from 21 commercially available seroconversion panels: 3 panels from BBI (West Bridgewater, MA), 5 panels from NABI (Boca Raton, FL), and 13 panels from Zeptometrix (Buffalo, NY). These samples were divided into two groups: group 1 comprised plasma samples obtained <65 days after the last anti-HCV-negative result, and group 2 comprised samples obtained >65 days after the last anti-HCV-negative result. A total of 362 plasma and serum samples from anti-HCV-positive (recombinant immunoblot assay confirmed, with reactivity to all bands) and HCV RNA-positive first-time blood donors (65 samples from BBI and 297 samples from the American Red Cross, each representing a unique individual) comprised group 3. Samples from 21 anti-HCV confirmed positive but HCV RNA-negative blood donors with resolved infection (obtained from Blood Systems Research Institute, San Francisco, CA) comprised group 4. All samples were tested by an ELISA (ELISA-ANTI-HCV; RPC Diagnostic Systems, Nizhniy Novgorod, Russia) to determine the presence of anti-HCV IgG.
AI assay.
The detection of antibody avidity was based on an indirect ELISA method. Four recombinant HCV proteins were used. They were derived from (i) the core region (from amino acid positions 1 to 100) of a genotype 1b HCV strain, (ii) the NS3 region (positions 1356 to 1459) of a genotype 1a HCV strain, (iii) the NS3 region (positions 1192 to 1459) of a genotype 1b HCV strain, and (iv) a mosaic protein containing immunodominant regions of the NS4 protein (from positions 1691 to 1710, 1712 to 1733, and 1921 to 1940) originating from HCV belonging to genotypes 1, 2, 3, and 5 (3). The proteins were obtained as a gift from Diagnostic Systems, Inc. (Nizhniy Novgorod, Russia). All proteins were expressed in Escherichia coli as a chimera with glutathione S-transferase and were purified using Sepharose-affinity chromatography (Pharmacia Biotech, Inc., Piscataway, NJ). Purified antigens were diluted in phosphate-buffered saline (PBS) containing 5 M urea (pH 7.1). Individual wells of Nunc Poly Sorp plates (Nunc, Inc., Denmark) were coated with 0.1-ml volumes of a mixture containing 1 μg/ml of core protein, 1 μg/ml of NS3 protein genotype 1a, 2 μg/ml of NS3 protein genotype 1b, and 2 μg/ml of NS4 mosaic protein. Plates were incubated overnight at room temperature.
For the determination of AI, a test sample was diluted 1:100 in dilution buffer containing 2% normal goat serum, 0.5 M urea, 0.5 M NaCl, and PBS-Tween 20. One hundred microliters of the sample was applied to the HCV antigen-coated well in duplicate for 1 h at 37°C. After being washed with PBS-Tween 20, one of the duplicate wells was treated with 100 μl of physiological saline (0.85% NaCl) and the second well with 8 M urea for 10 min at 37°C. The wells were washed and then incubated for 30 min at 37°C with 100 μl of peroxidase-labeled goat anti-human IgG (Pierce Biotechnology, Inc., Rockford, IL). Finally, after being washed, the wells were incubated with 3,3′,5,5′-tetramethylbenzidine for 30 min at room temperature. After the reaction was stopped with 100 μl of 2 M H2SO4, the optical density (OD) at 450 nm was measured. AI was determined as the ratio of the OD value obtained following urea treatment divided by the OD obtained without urea treatment, expressed as a percentage.
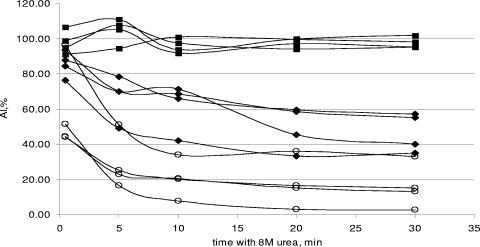
Effect of urea treatment time on AI.
Four specimens from each group were tested using different contact times with 8 M urea: 30 s, 2 min, 5 min, 10 min, 20 min, and 30 min (Fig. 1). For samples from patients with chronic infection (group 3), no difference between the AI values obtained after different contact times was observed. However, the AI values observed for the seroconversion panel groups (groups 1 and 2) sharply declined during the first 10 min and then stabilized thereafter. Group 4 samples showed AI values that declined throughout all time periods tested. The largest differences in AI values were observed between groups 3 and 1 and between groups 3 and 2 after a treatment time of 10 min. This duration was considered the optimal treatment time.
FIG. 1.
Effect of urea treatment time on AI. Squares represent samples from anti-HCV- and RNA-positive blood donors (group 3), diamonds represent sera from patients with resolved infection (group 4), and open circles represent plasma specimens from seroconversion panels (groups 1 and 2).
Variability of the assay.
The coefficients of intraassay and interassay variation, which were evaluated by testing, respectively, nine replicates of eight sera in the same run and nine replicates of eight sera in nine different runs, were 9.4% and 12.3%, respectively. Student's t test was used to estimate the significance of the difference between the mean values.
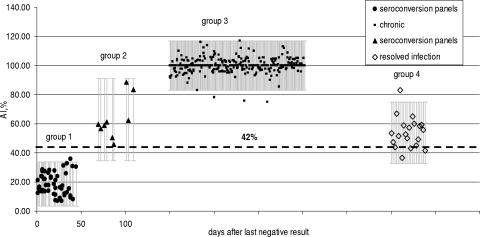
Differences in AI values among seroconversion samples obtained <65 days and >65 days after the last anti-HCV-negative result, chronically infected blood donors, and blood donors with resolved HCV infection.
Figure 2 shows the results of the AI values obtained for samples from all four groups. Time zero along the x axis denotes the time point at which the last specimen of a seroconversion panel tested negative for anti-HCV. The mean AI value obtained for specimens in group 1 was 18.6%, with the 95% confidence limits (CL) being 3.5% to 33.7%. Group 2 samples showed a mean AI value of 63% (95% CL, 34.7% to 91.3%). Thus, the AI for group 2 was larger than that for group 1, reflecting the maturation in IgG avidity that occurs within this time frame. For group 3, which represents samples obtained from anti-HCV- and HCV RNA-positive blood donors with chronic HCV infection, no data regarding prior anti-HCV antibody results were available. Hence, the time of last known anti-HCV-negative result is not known for this group, but it is assumed that samples in group 3 are not new infections. The mean AI for group 3 was 100% (95% CL, 83.1% to 116.9%). The data are compatible with data observed from previous studies showing increased avidity of HCV antibodies over time following seroconversion (11, 16). Similar data have been reported for dengue patients (6). Samples from blood donors with resolved infection, defined as anti-HCV confirmed positive but negative for HCV RNA (group 4), showed a mean AI of 54% (95% CL, 32.8% to 75%).
FIG. 2.
AI differences among four study groups. Group 1, plasma specimens from seroconversion panels obtained within days 0 to 45 after the last negative result (n = 51) (representing primary infection); group 2, plasma specimens from seroconversion panels obtained more than 65 days after the last negative result (n = 9) (representing maturating primary infection); group 3, plasma and serum specimens from anti-HCV- and HCV RNA-positive blood donors (n = 365) (representing chronic infection); and group 4, serum specimens from blood donors with resolved HCV RNA-negative infection (n = 22) (representing past infection). For groups 3 and 4, the numbers of days after the last negative result are unknown. The dashed horizontal line at an AI of 42% represents a statistically derived cutoff value.
The horizontal line shown in Fig. 2, corresponding to an AI of 42%, represents a statistically derived cutoff value to distinguish acute HCV infection from chronic and from resolved infection. This cutoff value was calculated as the mean AI value of acute infections (group 1) plus 3 standard deviations of the mean. This cutoff value indicates that all samples in group 1 are acute-phase specimens, that all members of group 2 are late-acute-phase samples (i.e., >65 days after the last anti-HCV-negative result), that all members of group 3 are readily distinguishable from members of group 1 (P < 0.001), and that group 4 specimens (resolved infections) cannot be distinguished from acute-phase specimens by using the AI alone.
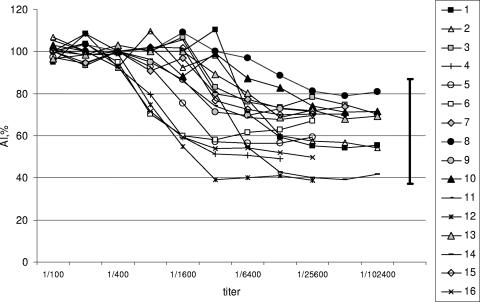
Dependence of AI values on IgG antibody titer.
Samples from blood donors with resolved infections may have yielded AI values lower than those for group 3 because of a lower titer of circulating anti-HCV IgG, reflecting an absence of antigenic stimulation (9, 11). To investigate if the titer of IgG influences AI measurements, AI values of serially diluted samples from 16 randomly selected anti-HCV-seropositive blood donors from group 3 were determined. The anti-HCV titers ranged from 1:100 to 1:102,400. High AI values were maintained down to a 1:400 dilution, thereafter declining with dilution (Fig. 3). For each test sample, the lowest AI value was higher than the AI value in samples obtained from group 1 (P < 0.001) (Fig. 2). The AI values obtained from the group 3 dilution studies were comparable to those for group 4. Therefore, these data suggest that the lower AI values obtained from blood donors with resolved infections (group 4) may be due to lower levels of circulating IgG anti-HCV antibody.
FIG. 3.
Dependence of AI on antibody titer. Serial dilutions of specimens from 16 chronically infected blood donors at dilutions from 1:100 to the lowest titer at which seropositivity for anti-HCV became detectable by ELISA.
Summary and proposal for clinical use of avidity testing.
This investigation confirms that there is a significant difference between the avidity of anti-HCV IgG in infected persons with primary incident infections (group 1) and that of persons who are chronically infected (group 3). However, the differences in avidity in samples from blood donors with resolved infection (group 4) and samples from late-acute-phase primary infections (group 2) are less distinct. It is important to note that testing for HCV RNA in individuals positive for anti-HCV is a routine practice used to distinguish between active and resolved infections. The detection of HCV RNA would be a necessary test in samples demonstrating low avidity to identify active infection before initiating antiviral treatment. Prospective studies are being designed to apply this new test in various epidemiological settings.
Acknowledgments
We acknowledge funding from the CDC through an RO1 grant that funded follow-up of Blood Systems Research Institute resolved cases.
Footnotes
Published ahead of print on 22 August 2007.
REFERENCES
- 1.Brillanti, S., C. Masci, P. Ricci, M. Miglioli, and L. Barbara. 1992. Significance of IgM antibody to hepatitis C virus in patients with chronic hepatitis C. J. Hepatol. 15:998-1001. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 1998. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Morb. Mortal. Wkly. Rep. 47(RR-19):1-39. [Google Scholar]
- 3.Chang, J. C., B. Ruedinger, M.-E. Cong, S. Lambert, E. Lopareva, M. Purdy, B. P. Holloway, D. L. Jue, B. Ofenloch, H. A. Fields, and Y. E. Khudyakov. 1999. Artificial NS4 mosaic antigen of hepatitis C virus. J. Med. Virol. 59:437-450. [PubMed] [Google Scholar]
- 4.Chau, K. H., G. J. Dawson, I. K. Mushahwar, R. A. Gutierrez, R. G. Johnson, R. R. Lesniewski, L. Mattsson, and O. Weiland. 1991. IgM-antibody response to hepatitis C virus antigens in acute and chronic post-transfusion non-A, non-B hepatitis. J. Virol. Methods 35:343-352. [DOI] [PubMed] [Google Scholar]
- 5.Chen, P. J., J. T. Wang, L. H. Hwang, Y. H. Yang, C. L. Hsieh, J. H. Kao, J. C. Sheu, M. Y. Lai, T. H. Wang, and D. S. Chen. 1992. Transient immunoglobulin M antibody response to hepatitis C virus capsid antigen in posttransfusion hepatitis C: putative serological marker for acute viral infection. Proc. Natl. Acad. Sci. USA 89:5971-5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Souza, V. A. U. F., S. Fernandes, E. S. Araujo, A. F. Tateno, O. M. N. P. F. Oliveria, R. D. R. Oliveira, and C. S. Pannuti. 2004. Use of an immunoglobulin G avidity test to discriminate between primary and secondary dengue virus infections. J. Clin. Microbiol. 42:1782-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisen, H. N., and G. W. Siskind. 1964. Variations in affinities of antibodies during the immune response. Biochemistry 3:996-1008. [DOI] [PubMed] [Google Scholar]
- 8.Gray, J. J., and T. G. Wreghitt. 1989. Immunoglobulin G avidity in Epstein-Barr virus infection in organ transplant recipients. Serodiagn. Immunother. Infect. Dis. 3:389-393. [Google Scholar]
- 9.Hedman, K., and I. Seppala. 1988. Recent rubella virus infection indicated by a low avidity of specific IgG. J. Clin. Immunol. 8:214-221. [DOI] [PubMed] [Google Scholar]
- 10.Hofer, H., T. Watkins-Riedel, O. Janata, E. Penner, H. Holzmann, P. Steindl-Munda, A. Gangl, and P. Ferenci. 2003. Spontaneous viral clearance in patients with acute hepatitis C can be predicted by repeated measurements of serum viral load. Hepatology 37:60-64. [DOI] [PubMed] [Google Scholar]
- 11.Kanno, A., and Y. Kazuyama. 2002. Immunoglobulin G antibody avidity assay for serodiagnosis of hepatitis C virus infection. J. Med. Virol. 68:229-233. [DOI] [PubMed] [Google Scholar]
- 12.Larghi, A., M. Zuin, A. Crosignani, M. L. Ribero, C. Pipia, P. M. Battezzati, G. Binelli, F. Donato, A. R. Zanetti, M. Podda, and A. Tagger. 2002. Outcome of an outbreak of acute hepatitis C among healthy volunteers participating in pharmacokinetics studies. Hepatology 36:993-1000. [DOI] [PubMed] [Google Scholar]
- 13.Lehmann, M., M. F. Meyer, M. Monazahian, H. L. Tillmann, M. P. Manns, and H. Wedemeyer. 2004. High rate of spontaneous clearance of acute hepatitis C virus genotype 3 infection. J. Med. Virol. 73:387-391. [DOI] [PubMed] [Google Scholar]
- 14.Lu, S.-N., H.-D. Tung, T.-M. Chen, C.-M. Lee, J.-H. Wang, C.-H. Hung, C.-H. Chen, and C.-S. Changchien. 2004. Is it possible to diagnose acute hepatitis C virus (HCV) infection by a rising anti-HCV titer rather than by seroconversion? J. Viral Hepat. 11:563-570. [DOI] [PubMed] [Google Scholar]
- 15.Marcellin, P. 1999. Hepatitis C: the clinical spectrum of the disease. J. Hepatol. 31:9-16. [DOI] [PubMed] [Google Scholar]
- 16.Ward, K. N., W. Dhaliwal, K. L. Ashworth, E. J. Clutterbuck, and C. G. Teo. 1994. Measurement of antibody avidity for hepatitis C virus distinguishes primary antibody responses from passively acquired antibody. J. Med. Virol. 43:367-372. [DOI] [PubMed] [Google Scholar]
- 17.Wawrzynowicz-Syczewska, M., J. Kubicka, Z. Lewandowski, A. Boron-Kaczmarska, and M. Radkowski. 2004. Natural history of acute symptomatic hepatitis type C. Infection 32:138-143. [DOI] [PubMed] [Google Scholar]