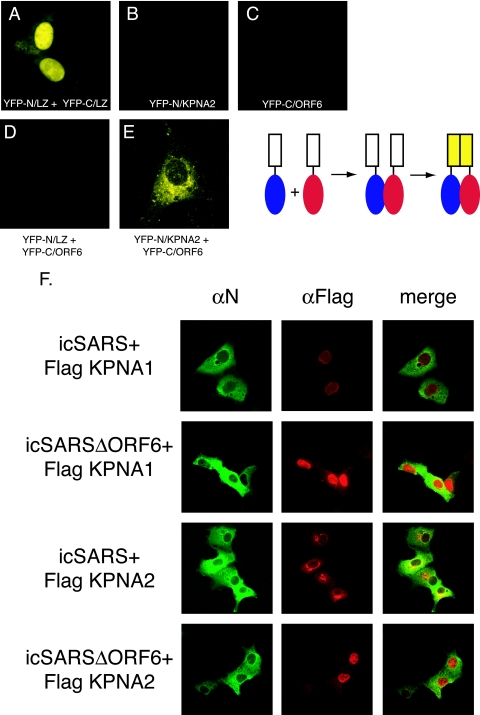
FIG. 4.
Interaction between KPNA2 and ORF6 in vivo. The top panel shows a schematic of the split YFP assay. The cargo is fused to each half of YFP. When the cargo interacts, the YFP halves are brought together to re-form and fluoresce. Vero cells were transfected with YFP plasmids, and 24 h after transfection, YFP fluorescence was visualized on a confocal microscope using a YFP filter. (A) YFP-N/leucine zipper (LZ) and YFP-C/leucine zipper (positive controls). (B) YFP-N/KPNA2. (C) YFP-C/ORF6. (D) YFP-N/leucine zipper plus YFP-C/ORF6. (E) YFP-N/KPNA2 and YFP-C/ORF6. (F) Karyopherin localization during SARS infection. Vero cells were transfected with Flag-tagged KPNA1 or KPNA2 24 h prior to infection with either icSARS or icSARSΔORF6. Cells were fixed in 4% PFA prior to antibody staining. SARS mouse anti-N antibody (αN) was used to localize SARS-infected cells, and rabbit anti-Flag antibody was used to localize karyopherins. Mouse anti-N antibody was visualized with Alexa Fluor 488-conjugated secondary antibody, and anti-Flag antibody was visualized with Alexa Fluor 546-conjugated secondary antibody.