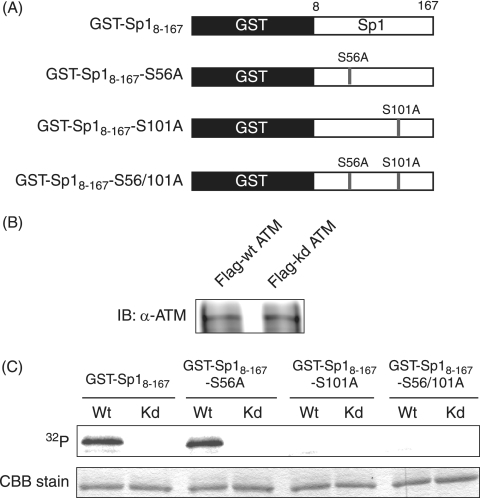
FIG. 8.
ATM phosphorylates Sp1 at Ser-101 but not Ser-56 in vitro. (A) Schematic diagram of GST fusion proteins of truncated Sp1 and identical fragments with one or both of Ser-56 and Ser-101 mutated to alanine. (B) Lysates of 293T cells transfected with plasmid expressing Flag-tagged wild-type ATM (Flag-wt ATM) or kinase-dead ATM (Flag-kd ATM) were immunoprecipitated with anti-Flag antibody. Immunocomplexes of Flag-wt ATM or Flag-kd ATM were resolved on SDS-10% PAGE gel and subjected to immunoblot analysis with anti-ATM antibody. (C) IP-kinase assays. GST-Sp18-167, GST-Sp18-167-S56A, GST-Sp18-167-S101A, and GST-Sp18-167-S56/101A were expressed in E. coli, purified, and used as substrates for IP-kinase assays. Immunocomplexes containing Flag-wt ATM (Wt) or Flag-kd ATM (Kd) protein were each incubated with purified GST-Sp18-167, GST-Sp18-167-S56A, GST-Sp18-167-S101A, or GST-Sp18-167-S56/101A as substrates in the presence of [γ-32P]ATP. Samples were resolved by SDS-10% PAGE, followed by autoradiography. The amounts of each GST fusion protein were confirmed by Coomassie brilliant blue (CBB) staining.