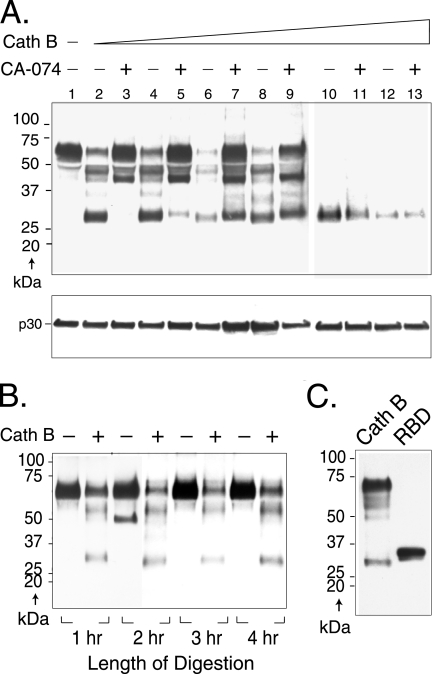
FIG. 7.
Cathepsin B (Cath B) cleaves virion SU at specific sites in a dose-dependent manner. (A) Aliquots of replication-competent Moloney MLV purified by high-speed ultracentrifugation for 90 min were incubated with increasing amounts of cathepsin B for 1 h in acetate buffer, pH 5.5, in the presence or absence of 100 μM CA-074Me and then immunoblotted to anti-SU antiserum (top), and the immunoblot was subsequently stripped and reprobed with anti-CA antiserum (bottom). Lane 1, no cathepsin B; lanes 2 and 3, 1.25 μg/ml; lanes 4 and 5, 12.5 μg/ml; lanes 6 and 7, 68 μg/ml; lanes 8 and 9, 125 μg/ml; lanes 10 and 11, 250 μg/ml; lanes 12 and 13, 375 μg/ml cathepsin B. Similar results were obtained in a second independent experiment. (B) Virus was incubated in the presence or absence of cathepsin B in buffer, pH 5.5. Aliquots were removed at hourly intervals through 4 h, and digestion was terminated as described in Materials and Methods. Samples were analyzed by immunoblot analysis to anti-SU antiserum. (C) The RBD of SU is similar in antiserum reactivity and molecular mass to the 28-kDa fragment. Purified recombinant RBD (residues 1 to 236 plus 6 additional residues remaining from an affinity purification tag) from the closely related Friend MLV was immunoblotted to the anti-SU antiserum. For comparison, a sample of Moloney MLV incubated with cathepsin B (68 μg/ml for 1 h) as described above was included.