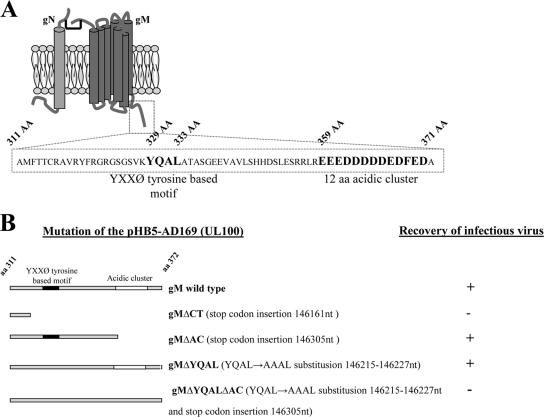
FIG. 2.
Proposed model of the gM/gN complex of HCMV and mutations introduced into the cytoplasmic tails of gMs of recombinant viruses. (A) The HCMV glycoprotein gM (UL100) cytoplasmic tail contains the tyrosine-based motif YXXØ and a cluster of acidic amino acids. A cartoon depicting the predicted structure of gM complexed with gN (46) is shown. gM is a type III glycoprotein with a predicted seven transmembrane domains. Sequence analysis of the predicted cytoplasmic domain of gM (aa 311 to 372) revealed two conserved trafficking motifs, (i) the tyrosine-based motif YXXØ and (ii) a cluster of 12 acidic amino acids at the carboxyl terminus of the protein. The amino acid sequences of both predicted motifs are depicted in boldface, and the amino acid positions are listed above the sequence. (B) Cartoon of the gM (UL100) cytoplasmic-tail mutations that were introduced into recombinant viruses. Note that infectious virus was not recovered from the BAC in which the predicted carboxyl-terminal cytoplasmic domain of gM was deleted by insertion of a translational stop codon or from the BAC in which both trafficking motifs were mutated simultaneously. The gM KO BAC was repaired using a wild-type UL100 fragment, and the growth kinetics of the recovered virus was identical to those of the AD169 wild type.