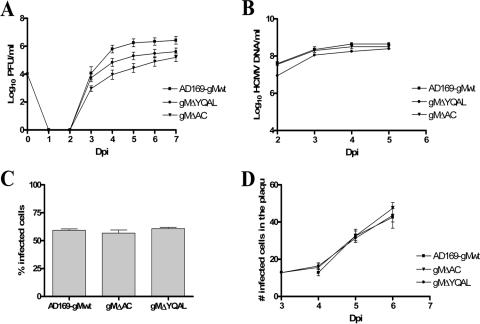
FIG. 3.
Kinetics of the growth, DNA replication, cell entry, and cell-to-cell spread of recombinant viruses containing mutations in the cytoplasmic domain of gM. (A) Growth kinetics in HFs of the wild-type HCMV compared with those of gMΔAC and gMΔYQAL recombinant viruses. HFs were infected with viruses at an MOI of 0.1, and total cultures (supernatant and cells) in 1.5 ml medium were harvested at the indicated time points p.i. (Dpi, days p.i.). The cell supernatants and disrupted cells were combined and assayed for the amounts of infectious particles, using a fluorescence-based infectivity assay (2). The results are expressed as log10 infectious units/ml of sample. The error bars indicate standard errors. (B) DNA replication assay. HFs were infected with the wild-type and recombinant gMΔAC and gMΔYQAL viruses at an MOI of 1.0. Infected cells were harvested at the indicated time points p.i., and total DNA was extracted. The HCMV genomic DNA copies were quantified using real-time PCR with primers specific for the HCMV glycoprotein gB (63). The results are expressed as log10 HCMV genome copies/ml of sample. (C) Cell entry assay. HFs plated on 13-mm glass coverslips in a 24-well plate were infected with wild-type and recombinant gMΔAC and gMΔYQAL viruses at an MOI of 1.0 and at 4°C for 1 h. The cells were then warmed to 37°C with prewarmed medium, and the coverslips were harvested at time zero. No IE-1 expression was detected at time zero. Three hours p.i., a second set of coverslips were fixed in 4% paraformaldehyde and stained with anti-IE-1 antibody to detect the number of cells expressing the IE-1 protein of HCMV. The total number of cells was estimated by Hoechst staining of host cell nuclei. The results are expressed as the percent IE-1-positive cells per 250 cells counted 3 h after being warmed to 37°C. (D) Mutations in the cytoplasmic domain of gM do not inhibit the cell-to-cell spread of HCMV. HFs were plated on 13-mm glass coverslips and then infected with wild-type HCMV AD169 or gMΔAC or gMΔYQAL recombinant virus at the same MOI of 0.1. Three hours after infection, the cells were overlaid with medium containing 0.3% low-melting-point agarose and allowed to solidify at room temperature. At 3 to 7 days p.i., as indicated, the cells were fixed in 4% paraformaldehyde and stained with antibody specific for the IE-1 antigen, as well as with Hoechst nuclear dye. The number of IE-1-positive cells per infectious focus was determined, and the mean and standard error of the number of IE-1-positive cells per focus were plotted versus the day p.i.