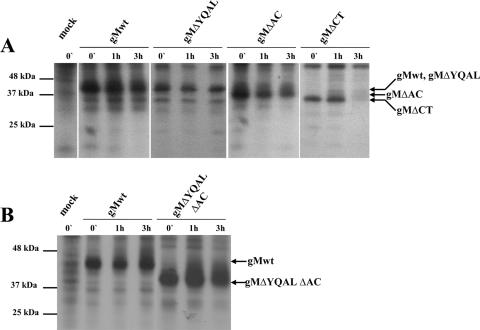
FIG. 4.
The stability of the gM mutant protein gMΔCT is decreased. Pulse-chase analysis of the wild-type gM (gMwt) (UL100) and gMΔYQAL, gMΔAC, and gMΔCT recombinant proteins (A) and gMΔYQALΔAC (B) was carried out as described in Materials and Methods. Briefly, HEK 293T cells were transfected with plasmids encoding carboxyl-terminally myc-tagged wild-type gM, gMΔYQAL, gMΔAC, gMΔCT, or gMΔYQALΔAC, together with a plasmid encoding a carboxyl-terminally FLAG-tagged gN. A nontransfected control cell culture was included in the analysis. Following radiolabeling with [35S]methionine/cysteine as described in Materials and Methods, the cells were lysed at different time points and the myc-tagged gM proteins were immunoprecipitated using anti-Myc antibodies conjugated to magnetic beads as previously described (46). Equivalent amounts of the immunoprecipitated proteins were analyzed by SDS-PAGE and fluorography. Molecular mass markers are shown on the left, and the arrows on the right point to migrations of gM-specific proteins.