Fig. 4.
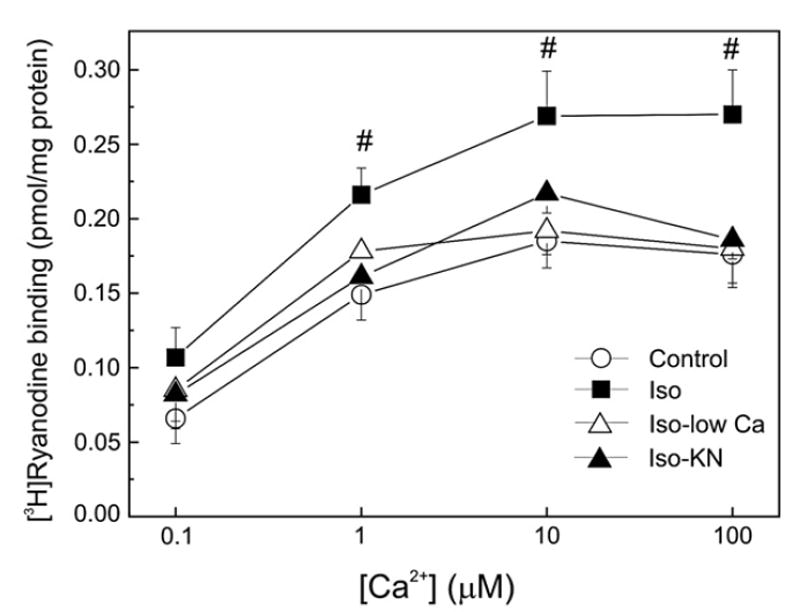
[3H]-ryanodine binding in SR vesicles isolated from treated hearts. Ca2+ dependence of [3H]-ryanodine binding in cardiac SR vesicles isolated from hearts perfused in the absence (Control) and the presence of 300 nM isoproterenol (Iso) and in the presence of Iso, either with low [Ca]o plus nifedipine (Iso-low Ca) or with 5 μM KN-93 (Iso-KN). Isoproterenol increased [3H]-ryanodine binding. This increase did not occur in the presence of KN-93 or low [Ca]o plus nifedipine. Of note, at 300 nM isoproterenol, the decrease in Ca2+ influx to the cell or the inhibition of CaMKII did not affect the phosphorylation of Ser2809 site but significantly decreased the phosphorylation of Ser2815 site (see Fig. 2). Neither KN-93 nor low [Ca]o plus nifedipine modified [3H]-ryanodine binding in the absence of isoproterenol. #p<0.05 with respect to control hearts.
