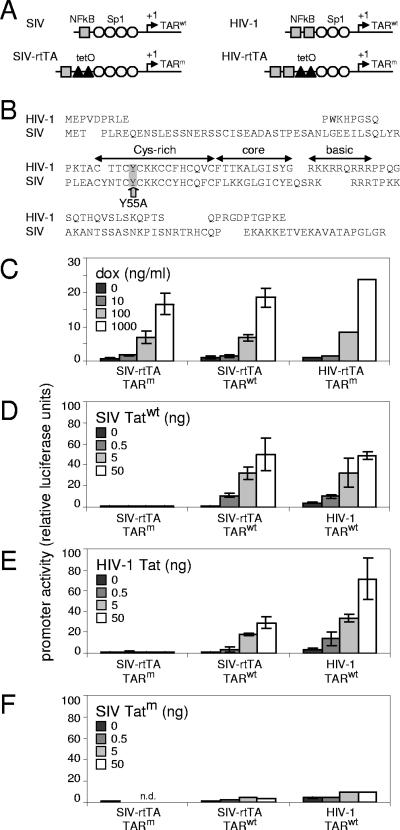
FIG. 3.
Expression from the SIV-rtTA LTR promoter is controlled by DOX. (A) To turn the SIV LTR into a DOX-inducible promoter, we mutated TAR and introduced two tetO elements between the NF-κB and Sp1 binding sites. This arrangement had previously been shown to be effective in the DOX-dependent HIV-rtTA variant. (B) Mutation of Tat. The SIVmac239 Tat protein consists of 130 amino acids and has a modular structure similar to that of the HIV-1 Tat protein, which consists of 86 to 101 amino acids (depending on the viral isolate). Both proteins have a transcription activation domain that can be subdivided in an N-terminal acidic domain, a cysteine-rich domain, a central core domain, and an RNA-binding domain consisting of a stretch of positively charged amino acids and therefore termed the basic domain. For the construction of HIV-rtTA, we previously inactivated HIV-1 Tat through the introduction of a tyrosine-to-alanine substitution at position 26 (Y26A) in the cysteine-rich domain. This cysteine-rich domain is highly conserved, and the tyrosine at position 26 in HIV-1 Tat corresponds to the tyrosine at position 55 in SIVmac239 Tat. We therefore introduced the Y55A mutation in the SIVmac239 Tat open reading frame. This mutation did not affect any other gene or known underlying sequence element. (C) C33A cells were transfected with a plasmid in which the expression of firefly luciferase was controlled by the LTR promoter of either SIV-rtTA, SIV-rtTA-TARwt, or HIV-rtTA and an rtTA-expressing plasmid. Transfected cells were cultured with 0 to 1,000 ng/ml DOX. (D to F) Cells were transfected with a plasmid in which the expression of firefly luciferase was controlled by the LTR promoter of either SIV-rtTA, SIV-rtTA-TARwt, or HIV-1 and 0 to 50 ng of a plasmid expressing wild-type SIV Tat (D), HIV-1 Tat (E), or Y55A-mutated SIV Tat (F). The intracellular luciferase level, which reflects promoter activity, was measured at 2 days after transfection. The error bars represent the standard deviations for two to five experiments (n.d., not determined).