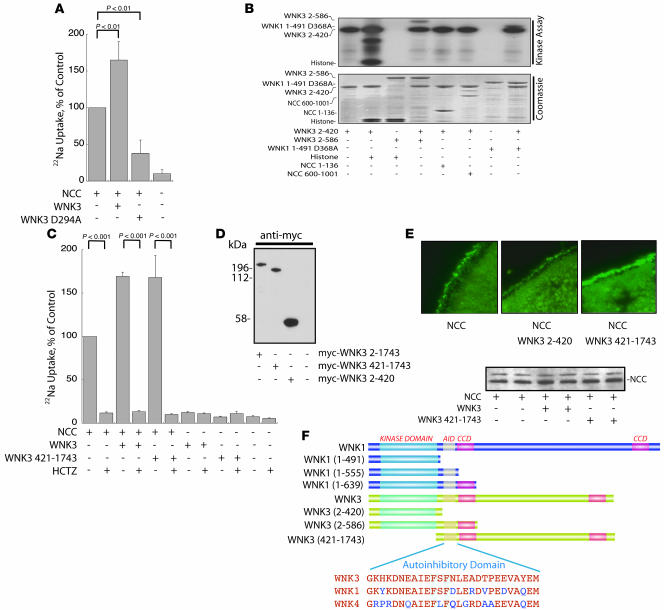
Figure 1. WNK3 stimulates but does not phosphorylate NCC.
(A) Full-length WNK3-stimulated Na uptake by Xenopus oocytes (expressed as percentage of Na uptake by oocytes injected with NCC alone), whereas kinase-dead WNK3 D294A downregulated Na uptake, compared with NCC alone. n = 4. (B) GST-WNK3 2–420 phosphorylated itself and the substrate histone, whereas GST-WNK3 2–586 was inactive. WNK3 2–420 also phosphorylated the kinase-inactive GST-WNK3 2–586 and GST-WNK1 1–491 D368A. WNK3 did not phosphorylate NCC, within either the N terminus (GST-NCC 1–136) or the C terminus (GST-NCC 600-1,001). Top: kinase assay; bottom: Coomassie-stained gel. Results are representative of experiments performed in triplicate. (C) The C terminus of WNK3 (WNK3 421–1,743) increased NCC activity in a manner similar to full-length WNK3. The NCC stimulation was inhibited by hydrochlorothiazide (HCTZ). Neither WNK3 alone nor WNK3 421–1,743 alone stimulated Na uptake in the absence of NCC. WNK3 2–420 had no effect on NCC activity (data not shown); n = 5. (D) WNK3 constructs were all expressed at the protein level (Western blot of Xenopus oocyte lysate). (E) WNK3 421–1,743, but not the kinase domain, WNK3 2–420, increased the abundance of NCC at the plasma membrane of oocytes, as detected by immunocytochemistry (results are representative of experiments performed in triplicate). Original magnification, ×400. Western blot of oocyte lysate showed no effect on total NCC. (F) Comparison of the domain structure of WNK1 and constructs employed in the present experiments. AID, autoinhibitory domain; CCD, coiled-coil domain. Sequences of autoinhibitory domains of WNK kinases are compared. Key phenylalanines shown to be essential for autoinhibition of WNK1 (24) are highlighted in blue.