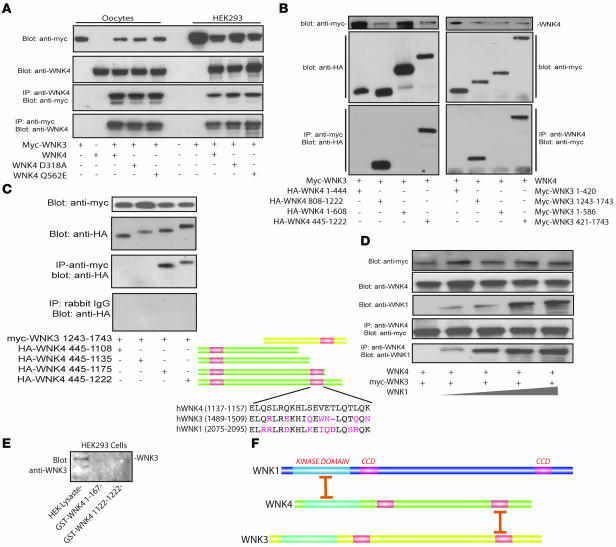
Figure 3. WNK3 associates with WNK4 and WNK1.
(A) WNK3 associated with WNK4, FHHt mutant WNK4 (WNK4 Q562E), and kinase-dead WNK4 (D318A) in Xenopus oocytes and HEK293t cells. (B) WNK3 and WNK4 associate within their C termini. Left: myc-WNK3 expressed with HA-tagged fragments of WNK4. Anti-myc antibody precipitated only WNK4 fragments that included the carboxyl terminal domain (WNK4 445–1,222 and 808–1,222). Right: WNK4 precipitated only WNK3 fragments that contain the carboxyl terminal domain (WNK3 421–1,743 and 1,243–1,743). (C) Identification of the WNK4 region involved in interaction with WNK3. Progressive truncation of the WNK4 carboxyl terminus identified a region between residues 1,135 and 1,175 as essential for interaction. This region encompasses the second WNK4 coiled-coil domain, as shown schematically. The coiled-coil domains of WNK1, -3, and -4 are compared. (D) WNK1, WNK3, and WNK4 formed protein complexes in Xenopus oocytes. Myc-WNK3 and WNK4 were expressed with increasing amounts of WNK1 cRNA. The WNK3/WNK4 expression ratio was 1:1. The WNK1/WNK3 and WNK1/WNK4 ratios were 0.25:1 to 2:1. Lysates were precipitated using an anti-WNK4 antibody and detected using anti-myc and anti-WNK1. Increasing expression of WNK1 did not dissociate the complex. (E) GST-WNK4 1,122–1,222, but not GST-WNK4 1–167, pulls down endogenous WNK3 from HEK293 cells. Endogenous WNK3 is present in cell lysate. Results are representative of experiments performed in triplicate. (F) Schematic comparing sites of association between WNK4 with WNK3 (these studies) and WNK4 with WNK1 (22).