Figure 1. Role of flanking amino acid residues in MHC class I and class II peptide biology.
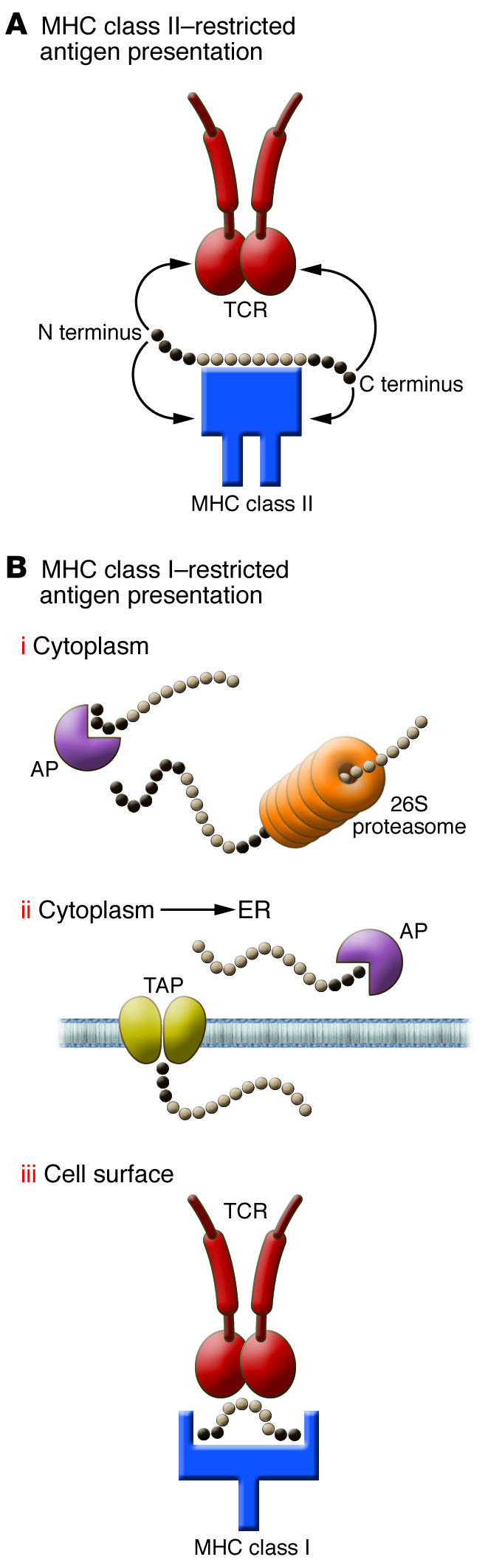
For MHC class II molecules (A), N and C terminal flanking residues protrude outside the MHC class II groove and are sometimes involved in MHC binding and/or TCR recognition. For MHC class I residues (B), amino acids N and C terminal of the 8- to 9-mer core peptide have multiple functions: (i) binding and processing by the proteasome and cytoplasmic aminopeptidases (AP); (ii) binding and transport into the ER by the TAP heterodimer and further digestion by ER aminopeptidases; and (iii) T cell recognition when a longer peptide is tethered by its extremities and bulging outside of the MHC class I groove. The observation made by Le Gall et al. (7) in this issue of the JCI is likely to be linked to steps i and ii, in which the N terminal sequence of the peptide will control the accessibility of aminopeptidases and the overall abundance of the final MHC class I peptide.
