Abstract
While the role of attention in determining the neural fate of unattended emotional items has been investigated in the past, it remains unclear whether bottom-up and top-down factors have differential effects in shaping responses evoked by such stimuli. To study the effects of bottom-up and top-down factors on the processing of neutral and fearful faces, we employed functional magnetic resonance imaging (fMRI) while participants performed attentional tasks that manipulated these factors. To probe the impact of top-down mechanisms on the processing of face distractors, target letters either had to be found among several distinct nontarget letters (attentional load condition) or among identical nontarget letters (baseline condition). To probe the impact of bottom-up factors, we decreased the salience of the targets by reducing their size and contrast relative to baseline (salience condition). Our findings revealed that bottom-up and top-down manipulations produced dissociable effects on amygdala and fusiform gyrus responses to fearful-face distractors when task difficulty was equated. When the attentional load of the main task was high, weaker responses were evoked by fearful-face distractors relative to baseline during the early trials. By contrast, decreasing target salience resulted in increased responses relative to baseline. The present findings suggest that responses evoked by unattended fearful faces are modulated by several factors, including attention and stimulus salience.
Keywords: attention, emotion, perception, facial expressions, fMRI
Introduction
During visual perception, it has been proposed that both task-relevant and task-irrelevant objects compete for limited processing resources (Bundesen, 1990; Desimone & Duncan, 1995; Grossberg, 1980). Because the processing capacity of the perceptual system is limited (Broadbent, 1958), selective attention to objects relevant to current behavior impacts on the processing of task-irrelevant distractors. Indeed, a fundamental issue in the study of selective attention concerns the extent to which distractors are processed (Deutsch & Deutsch, 1963; Treisman, 1960). In general, both bottom-up and top-down factors determine the extent of distractor processing (Bundesen, 1990; Desimone & Duncan, 1995; Grossberg, 1980). Bottom-up mechanisms reflect sensory stimulation, such as stimulus salience. Previous studies have demonstrated that if a salient distractor is presented in a display, the search time for a target will increase relative to the presence of a nonsalient distractor (Eltiti, Wallace, & Fox, 2005; Mounts, 2005; Theeuwes, 2005; Yantis & Jonides, 1990). The increase of the search time suggests that salience biases the processing in favor of the distractor and thereby impairs target performance. Distractor processing is also determined via top-down processes, such as attention, reflecting the requirements of the “main” task. For example, if the “load” of the main task is high, most resources will be applied towards its processing. As a result, few resources, or possibly none, will be available for the processing of task-irrelevant items outside the focus of attention (Lavie, 1995).
Fearful expressions serve as important social signals, potentially conveying the source of threat-related information. Psychophysiological studies have shown that participants exhibit fast, involuntary responses to threat-related stimuli (Globisch, Hamm, Esteves, & Öhman, 1999; Öhman, Esteves, & Soares, 1995). Because of such biological significance, several studies have suggested that task-irrelevant fearful faces are processed “automatically”, largely independent of attention (Anderson, Christoff, Panitz, De Rosa, & Gabrieli, 2003; Vuilleumier, Armony, Driver, & Dolan, 2001; Williams, McGlone, Abbott, & Mattingley, 2005). A competing line of research has suggested that the processing of emotion-laden information in general, and emotional faces in particular, is not immune to attentional manipulations. Findings from these studies suggest that when the main task is demanding, the processing of irrelevant emotion-laden items can be modulated by attention (Erthal et al., 2005; Holmes, Vuilleumier, & Eimer, 2003; Holmes, Winston, & Eimer, 2005; Pessoa, McKenna, Gutierrez, & Ungerleider, 2002; Pessoa, Padmala, & Morland, 2005).
While the role of attention in determining the neural fate of unattended emotional items has been investigated in the past, it remains unclear whether bottom-up and top-down factors have differential effects in shaping responses evoked by such stimuli. To study the effects of these factors on the processing of fearful faces, we employed functional magnetic resonance imaging (fMRI) while participants performed attentional tasks that manipulated these factors. Participants viewed displays containing a central, circular array of letters and two task-irrelevant faces presented peripherally to the left or right of fixation; faces could be either neutral or fearful (Fig. 1). In the present study, we operationally defined bottom-up and top-down manipulations as follows. To probe the impact of top-down mechanisms on the processing of the task-irrelevant faces, we manipulated the load of the main task: a target letter had to be found either among several distinct nontarget letters (attentional load condition; Fig. 1A) or among an array comprised of the same nontarget letter (baseline condition; Fig 1B). In the past, this target search task has been successfully used to manipulate attentional load (Lavie, 1995, 2005). To probe the impact of bottom-up factors, we manipulated stimulus salience by including a condition in which the letters of the central array were smaller and of reduced contrast (salience condition; Fig. 1C) relative to that in the baseline condition. This size/contrast manipulation also has been widely used to examine salience effects (Wolfe, 1998). Because salience is a relative property that depends on the relationship of one object with respect to other objects in a display (Fecteau & Munoz, 2006), the size/contrast manipulation was expected to affect the relative salience of the task-irrelevant faces (Mounts, 2005; Treisman & Gormican, 1988). Critically, our goal was to manipulate attentional load and stimulus salience (relative to baseline), while matching their task difficulty. We focused our analyses on two key structures that have been extensively probed in prior studies of emotional perception: the amygdala, a key node in the processing of emotion-laden information (Adolphs, Tranel, Damasio, & Damasio, 1994; Breiter et al., 1996; Young et al., 1995), and the fusiform gyrus, a structure important for the processing of faces (Haxby, Hoffman, & Gobbini, 2000; Kanwisher, McDermott, & Chun, 1997; Puce, Allison, Asgari, Gore, & McCarthy, 1996).
Figure 1.
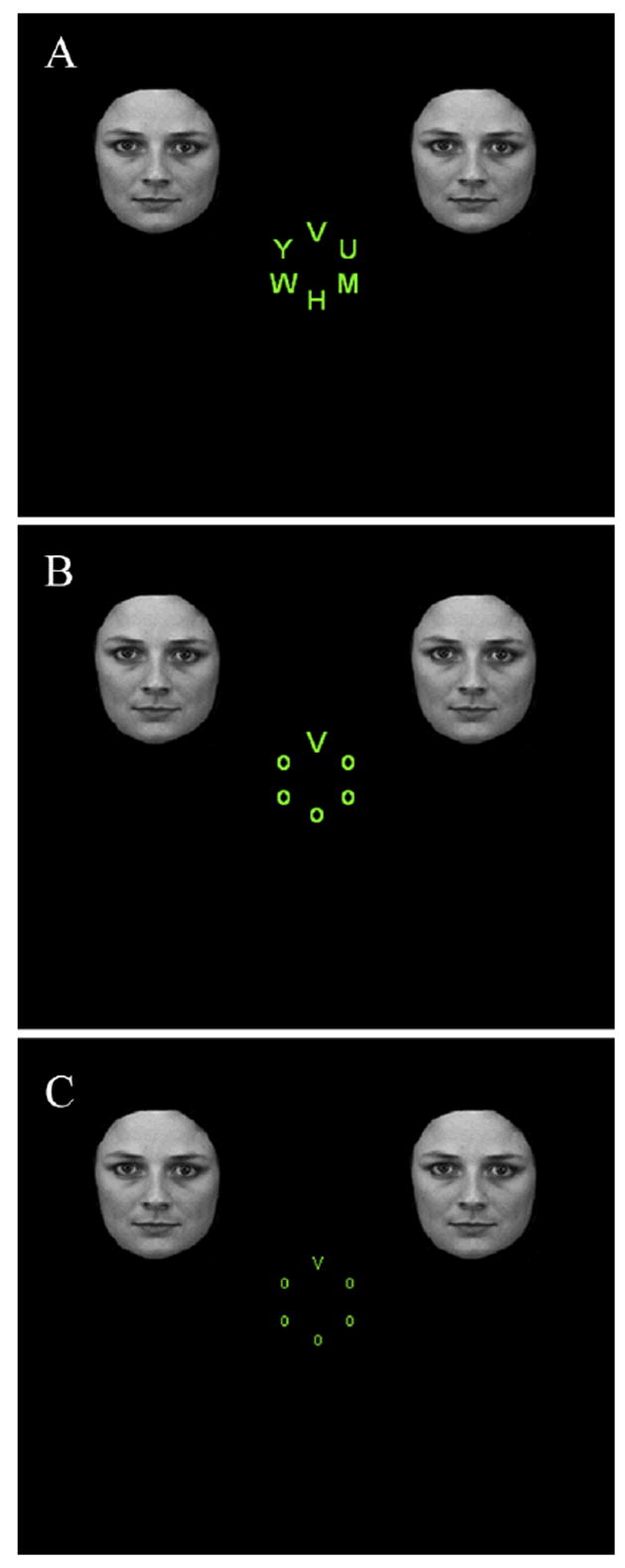
Sample stimulus displays containing face distractors for the (A) attentional load, (B) baseline, and (C) salience conditions (stimuli not drawn to scale).
Previous studies have shown that, in addition to stimulus valence, amygdala activity is modulated by non-emotional tasks (Drevets & Raichle, 1998). For instance, performing neutral, difficult attentional tasks per se has been shown to decrease amygdala activation relative to fixation (Pessoa et al., 2005). Thus, in the present study, in addition to being driven by faces, the amygdala could be driven by the letter-detection task itself, making the interpretation of evoked responses problematic. Accordingly, to factor out potential task effects, we employed three additional conditions that were identical to those discussed above, but that did not contain task-irrelevant, peripheral faces. By subtracting out the contribution of the tasks, we thus could isolate the contribution of face distractors to evoked responses.
Amygdala and fusiform gyrus responses are also known to exhibit rapid attenuation to repeatedly presented faces (Breiter et al., 1996; Fischer et al., 2003; Grill-Spector et al., 1999; Phillips et al., 2001; Williams et al., 2004; Wright et al., 2001), suggesting that the time course of the responses is dynamic. In addition, such attenuation has been found to be sensitive to task manipulations (Henson, Shallice, Gorno-Tempini, & Dolan, 2002; Ishai, Pessoa, Bikle, & Ungerleider, 2004). Such findings prompted us to split our experimental trials into early and late trials allowing us to probe how both valence and task effects depended on time (early vs. late).
As described above, our experiment included two experimental conditions that were designed to be comparable in terms of task difficulty, but which differed in terms of the type of task demands. The attentional load condition sought to increase task difficulty (relative to baseline) by taxing processing resources with the search task. The salience condition sought to increase task difficulty (relative to baseline) by degrading the sensory stimulus. Critically, because the distractor faces were kept constant across these conditions, our design allowed us to compare the effects of capacity vs. sensory demands (Lavie & De Fockert, 2003; Nakayama & Joseph, 1998; Norman & Bobrow, 1975), as imposed by the attentional load and salience conditions, respectively. Such comparison is important because part of the controversy surrounding the question of “automaticity” of amygdala responses has centered on whether or not the reported modulation of these responses (e.g., Pessoa et al., 2002) was due to attention or general task difficulty (Compton, 2003).
In summary, the goal of the present experiment was to investigate how bottom-up and top-down factors affect responses evoked by unattended emotional faces. The key experimental manipulations changed the nature of the relevant tasks in the center of the display without affecting peripheral, task-irrelevant face distractors. We hypothesized that increasing distractor salience (via decreasing target salience) would increase distractor processing, consistent with previous findings (Eltiti et al., 2005; Mounts, 2005; Theeuwes, 2005; Yantis & Jonides, 1990). In contrast, increasing the attentional load of the main task would decrease distractor processing, as suggested by prior work (Lavie, 1995, 2005). More generally, in the present study, we sought to investigate how multiple factors, including attention, stimulus salience, stimulus valence, and time, combine to generate responses in the human amygdala and fusiform gyrus, two key structures involved in the processing of emotional faces.
Method
Participants
Twenty, right handed participants (9 males; ages 19 to 29) without past neurological or psychiatric history took part in the study. All had normal or corrected-to-normal vision and gave informed consent according to procedures approved by the Institute Review Boards of both Brown University and Memorial Hospital of Rhode Island.
Stimuli and procedure
As shown in Figure 1, a stimulus display consisted of a target letter (V or N) subtending 0.7° of visual angle horizontally by 0.9° vertically in the baseline and the attentional load conditions. In the salience condition, the size of the target letter was reduced to 90–50% (see below) and its contrast to 35% relative to values in the baseline condition. The target letter appeared randomly but with equal probability in one of the six positions arranged in a circle centered at 1.5° from fixation. The other five positions were either occupied by the letter “O” (baseline and salience conditions), or by the five nontarget letters “H, U, M, Y, and W” (attentional load condition). Nontarget letters appeared in any one of the six positions randomly and equally often. Two identical face distractors (3° x 4°) portraying either fearful or neutral expression were simultaneously presented in the upper right and upper left quadrants of the display. The distance between the faces and fixation was 5° from center to center. Face stimuli were obtained form the Ekman series (Ekman & Friesen, 1976), the Karolinska Directed Emotion Faces (Lundqvist, Flykt, & Öhmann, 1998) and a set developed and validated by Ishai at NIMH (Bethesda, USA)(Ishai et al., 2004). Forty instances of identity-matched fearful and neutral faces were adopted. Most of the hair and non-facial contours were excluded from all faces. The same set of 80 faces was used in all three task conditions.
Participants completed 6–8 experimental runs. Each run consisted of six different blocks presented in random order: baseline, attentional load, salience blocks and 3 additional blocks with corresponding task conditions but displays that did not contain face distractors (no-face conditions). Therefore, our design was a hybrid design consisting of a block structure (task) and an event-related structure (facial expression) within the blocks containing faces. For analysis purposes, our design could be viewed as a 3 (task) x 2 (facial expression) design, in addition to 3 no-face conditions. In summary, a total of nine experimental conditions were employed: baseline with fearful-face distractors [BASE(fear)], baseline with neutral-face distractors [BASE(neutral)], baseline without face distractors [BASE(noface)], attentional load with fearful-face distractors [ATT(fear)], attentional load with neutral-face distractors [ATT(neutral)], attentional load without face distractors [ATT(noface)], salience with fearful-face distractors [SAL(fear)], salience with neutral-face distractors [SAL(neutral)], and salience without face distractors [SAL(noface)].
Blocks contained a total of ten trials. Each trial began with a 500-ms fixation cross at the centre of the screen, followed by a stimulus display lasting 100 ms. During all conditions, participants were instructed to maintain fixation centrally and to identify the target letter V or N as quickly and accurately as possible via a button press; they were instructed to ignore the faces in the conditions containing them. A new trial was initiated after a 2-s response window. Before each run, the stimulus size used in the salience condition with face distractors was adjusted based on the performance of the attentional load condition with faces in the previous run to match the task difficulty between the two conditions (hence the 90–50% size range); stimulus size became larger if accuracy was higher during the attentional load condition than during the salience condition in the previous run and vice versa. In brief, we adjusted the stimulus size in a run-by-run fashion to equate task difficulty across conditions.
Eye movement monitoring
Eye movements were monitored in 12 participants during scanning using infrared video-oculography (Resonance Technology, Inc., Northridge, CA, USA) integrated with the ViewPoint eye tracker system (Arrington Research, Inc., Scottsdale, AZ, USA). To assess deviations from fixation during each trial, horizontal and vertical eye position were determined for a 200-ms temporal window that started 50 ms prior to display onset and finished 50 ms after the display (thus, the window “bracketed” the 100-ms stimulus display). Eye movements relative to the central fixation that exceeded 1.5 degrees were considered to be a saccade.
MRI scanning
MR data was collected using a 1.5 Tesla Symphony Magnetom scanner (Siemens Medical Systems, Erlangen, Germany). A scanning session began with the acquisition of a high-resolution MPRAGE anatomical volume (TR = 1900 ms, TE = 4.15 ms, TI = 1100 ms, 1-mm isotropic voxels). Subsequently, a total of 561–688 functional images were obtained for each participant with a TR of 3000 ms and a TE of 38ms. Each image comprised 34 axial slices with slice thickness of 3.75 mm and in-plane resolution of 3.75 mm x 3.75 mm.
FMRI Data analysis
Data were analyzed using AFNI tools (Cox, 1996; http://afni.nimh.nih.gov/afni), unless indicated otherwise. The first four functional images were discarded to allow for equilibration effects. The data were slice-time corrected and motion corrected. Functional data were coregistered with the anatomical data and both were normalized to the standard space defined by the Montreal Neurological Institute (MNI) using the BET and FLIRT tools of the FSL package (http://www.fmrib.ox.ac.uk/fsl). The functional data were then spatially smoothed with an 8-mm Gaussian filter (full width at half maximum). For each participant, individual trials were modeled by a canonical hemodynamic response function. Experimental trials of each of the 9 experimental conditions were separated into early versus late halves in order to investigate time-related activity; thus, a total of 18 regressors of interest were generated to model each event type. Data were then analyzed using the general linear model to obtain parameter estimates of each regressor and to generate random-effects statistical maps resulting from linear contrasts between different event types.
As discussed in the Introduction, to factor out potential task effects, we initially subtracted parameter estimates of the no-face conditions from the corresponding conditions; thus, initially, we computed the following 12 contrast maps: [BASE(fear, early) - BASE(noface, early)], [(BASE(fear, late) – BASE(noface, late)], [BASE(neutral, early) - BASE(noface, early)], [BASE(neutral, late) – BASE(noface, late)], [ATT(fear, early) - ATT(noface, early)], [ATT(fear, late) - ATT(noface, late)], [ATT(neutral, early) - ATT(noface, early)], [ATT(neutral, late) - ATT(noface, late)], [SAL(fear, early) - SAL(noface, early)], [SAL(fear, late) - SAL(noface, late)], [SAL(neutral, early) - SAL(noface, early)], and [SAL(neutral, late) - SAL(noface, late)]. Subsequently, group analyses were performed based on these contrast maps with participants treated as a random factor.
In keeping with our a priori focus on amygdala and fusiform gyrus, the analyses were mainly focused on these two structures: amygdala (mean MNI coordinates of peak responses across all contrasts reported below: left, x = −20, y = −3, z = −22; right, x = 23, y = −4, z = −21) and the fusiform gyrus (left, x = −34, y = −60, z = −17; right, x = 33, y = −60, z = −16). In addition, given our anatomical focus, results in these two structures were reported when p < 0.01 (uncorrected) and cluster size ≥ 5, although most activation survived stricter thresholds. Note that our focus on the amygdala and fusiform gyrus also helps mitigate the multiple comparisons problem. When inspecting general, task-related activation across the brain, we adopted a threshold of p < 0.001 (uncorrected).
Results
Eye-movement data
Across all trials, very few saccades occurred during the 200-ms temporal window for the 12 participants for whom eye movements were monitored; on average 4 trials per participant (approximately 1% of the trials). A repeated-measures analysis of variance (ANOVA) revealed no significant difference in the number of saccades as a function of task conditions (baseline, attentional load, salience), the presence of face distractors (face, noface), or their interaction. An additional ANOVA was also conducted for conditions with face distractors [3 tasks (baseline, attentional load, salience) x 2 facial expressions (fear, neutral)]. No significant main effects or interactions were found.
FMRI data
General task activation in the absence of face distractors
Initially, we probed task-related activations by comparing the attentional load and salience conditions to the baseline condition when no face distractors were presented (trials were collapsed across the early and the late trials). Relative to baseline, the attentional load condition evoked increased activity in the superior parietal lobule (SPL) (left, x = −27, y = −62, z = 53; right; x = −28, y = −63, z = 50), inferior frontal gyrus (left, x = −46, y = 9, z = 26; right, x = 43, y = 5, z = 26), and frontal eye field (left, x = −30, y = −3, z = 57; right, x = 27, y = −2, z = 53). Relative to the baseline, the salience condition evoked increased activity in the left SPL only (x = −27, y = −57, z = 55).
Next, we examined task effects in the amygdala and the fusiform gyrus. Relative to baseline, weaker responses were observed in the bilateral amygdala during the salience and the attentional load conditions (Fig. 2A and Fig. 2B, left). In addition, weaker responses were evoked in the left amygdala during the attentional load condition relative to the salience condition (results not shown). For the fusiform gyrus, there was no significant difference between the baseline and the salience conditions, but stronger responses were observed during the attentional load relative to the baseline condition (Fig. 2B, right).
Figure 2.
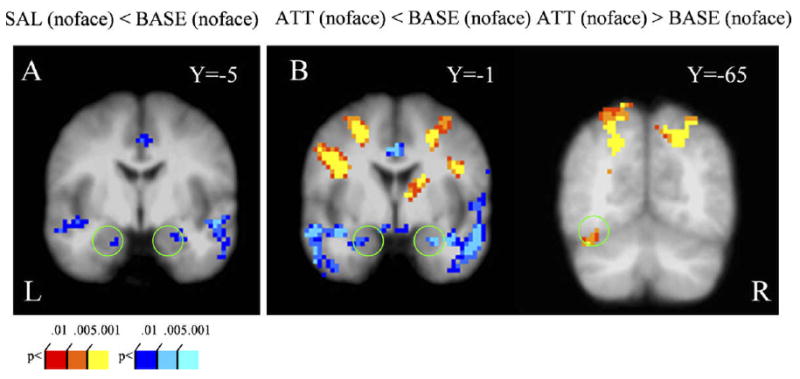
General task activation in the absence of face distractors. (A) Contrasting responses during the salience condition to those during the baseline revealed decreased amygdala activity. (B) Contrasting responses during the attentional load condition to those during baseline revealed decreased amygdala activity (left) but increased left fusiform gyrus activity (right). See Methods for contrast abbreviations.
A central goal of the present study was to examine distractor-related activation as a function of task condition. However, as evidenced by the above results, different tasks had differential impact on amygdala and fusiform gyrus responses when face distractors were absent. Accordingly, to account for differential task-related activation, responses during no-face conditions were subtracted from the responses in the corresponding conditions with fearful/neutral distractors (see Methods). For clarity, below, we provide labels for the contrasts involved and use the letter “a” to denote that responses were adjusted based on activity in the corresponding no-face conditions.
Time-related activity evoked by fearful and neutral distractors
To probe how distractor-related activity changed across time, we compared early relative to late responses. For both fearful and neutral face distractors, no time-related differential activation was observed in the amygdala and the fusiform gyrus during the baseline condition [BASEa(fear, early) ≈ BASEa(fear, late) and BASEa(neutral, early) ≈ BASEa(neutral, late)]. However, during the salience condition, greater activation in the right amygdala and the bilateral fusiform gyrus was found during the early trials relative to the late trials [SALa(fear, early) > SALa(fear, late) and SALa(neutral, early) > SALa(neutral, late); Fig. 3A and B], revealing that responses evoked by fearful and neutral distractors were attenuated over time during this condition. On the other hand, during the attentional load condition, weaker activation in the left amygdala and the right fusiform gyrus was found during the early trials relative to the late trials [ATTa(fear, early) < ATTa(fear, late) and ATTa(neutral, early) < ATTa(neutral, late); Fig. 3C and D], revealing that responses evoked by fearful and neutral distractors increased over time during this condition.
Figure 3.
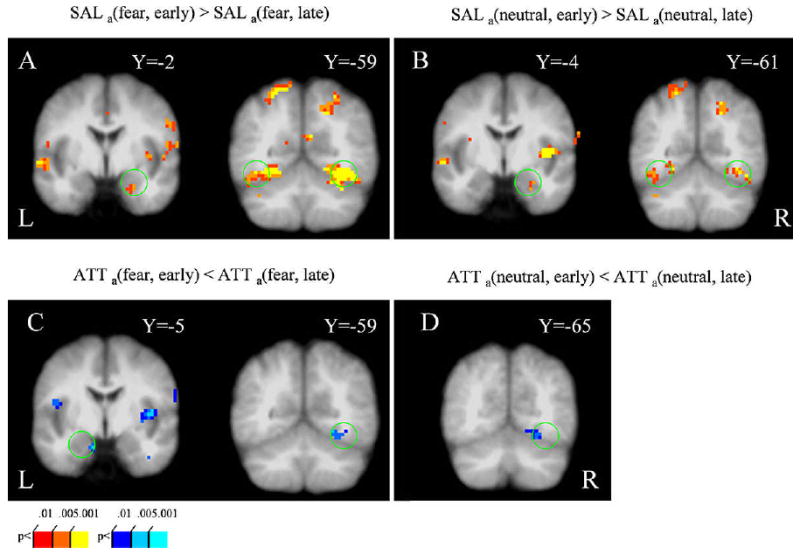
Time-related activity evoked by fearful and neutral distractors (early vs. late contrasts). During the salience condition, stronger responses to fearful (A) and neutral distractors (B) were observed in both the right amygdala (left) and the bilateral fusiform gyrus (right) during early trials. During the attentional load condition, weaker responses to fearful distractors were observed in both the amygdala and the fusiform gyrus (C) during early trials; weaker responses to neutral distractors were observed in the right fusiform gyrus during early trials (D). See Methods for contrast abbreviations.
Task effects on responses evoked by fearful and neutral distractors
Next, we investigated how the different task conditions modulated responses to face distractors. To minimize the contribution of time-related effects (see above), we focused our analysis on the early trials. Responses evoked by fearful-face distractors were stronger during the salience condition relative to the baseline condition in the right amygdala and the bilateral fusiform gyrus [SALa(fear, early) > BASEa(fear, early); Fig. 4A]. On the other hand, responses evoked by fearful-face distractors were actually weaker during the attentional load condition relative to the baseline condition in the left amygdala and the right fusiform gyrus [ATTa(fear, early) < BASEa(fear, early); Fig. 4B]. As expected from the above results, responses evoked during the salience condition relative to the attentional load condition were stronger in the right amygdala and the bilateral fusiform gyrus [SALa(fear, early) > ATTa(fear, early); Fig. 4C]. Analogous results as described for fearful-face distractors were also observed for neutral-face distractors (Fig. 4D, E, and F). To reiterate, during the early trials, responses evoked by both fearful and neutral distractors in the amygdala and the fusiform gyrus were stronger during the salience condition than in the baseline condition, which in turn, exhibited stronger activity than the attentional load condition.
Figure 4.
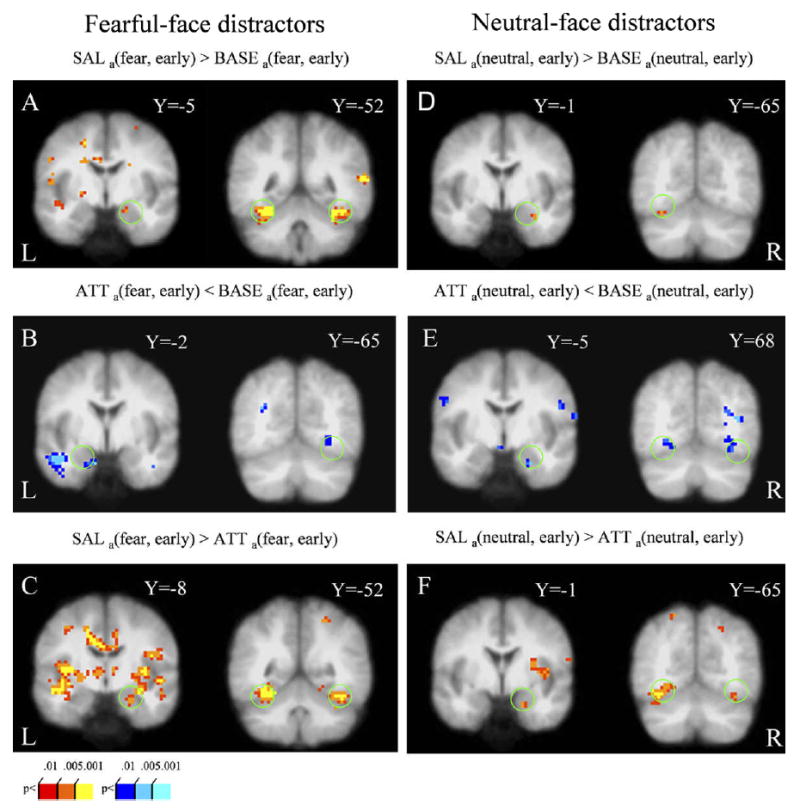
Task effects on responses evoked by fearful and neutral distractors during early trials. (A,B,C) Conditions involving fearful-face distractors. (A) Responses evoked by fearful and neutral distractors during the salience condition were stronger relative to baseline both in the right amygdala (left) and the bilateral fusiform gyrus (right). (B) Responses evoked by fearful distractors during the attentional load condition were weaker relative to baseline both in the left amygdala (left) and the right fusiform gyrus activity (right). (C) Responses evoked by fearful distractors during the salience condition were stronger relative to the attentional load condition in both the right amygdala (left) and the bilateral fusiform gyrus (right). (D,E,F). Conditions involving neutral-face distractors exhibited similar activation profiles. See Methods for contrast abbreviations.
Valence effects
We first examined valence effects during the early trials by contrasting neutral- and fearful-face conditions. Fearful-face distractors evoked stronger responses than neutral-face distractors in the left amygdala during both the baseline condition [BASEa(fear, early) > BASEa(neutral, early); Fig. 5A] and the attentional load condition [ATTa(fear, early) > ATTa(neutral, early); Fig. 5B]. During the salience condition, valence effects were observed in the right amygdala and bilateral fusiform gyrus [SALa(fear, early) > SALa(neutral, early); Fig. 5C]. No significant task by valence interaction was observed during the early trials.
Figure 5.
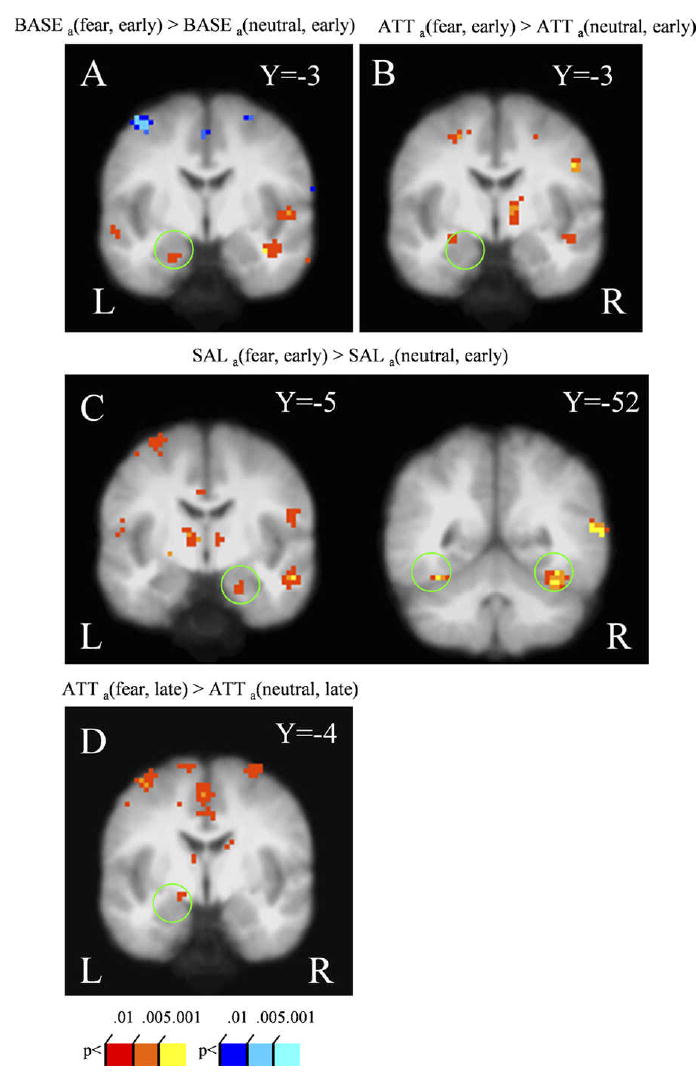
Valence effects. Stronger responses to fearful- relative to neutral-face distractors were observed in the amygdala during early trials for the (A) baseline (B) attentional load, and (C, left) salience conditions; for the latter condition, fusiform gyrus activity was also observed (C, right). During late trials, stronger responses to fearful- relative to neutral-face distractors were observed in the left amygdala for the attentional load condition (D). See Methods for contrast abbreviations.
Valence effects were also observed during the late trials. During the attentional load condition, stronger responses to fearful-face distractors relative to neutral-face distractors were observed in the left amygdala [ATTa(fear, late) > ATTa(neutral, late); Fig. 5D]. No significant differences were observed during the salience and baseline conditions.
Behavioral performance
Main effects of task and face distractors during early trials
Analogous to the analyses performed for the fMRI data, behavioral responses were also split into early and late trials. Here, we focused our analyses mainly on early trials (Fig. 6), although the results were equivalent when all trials were considered together.
Figure 6.
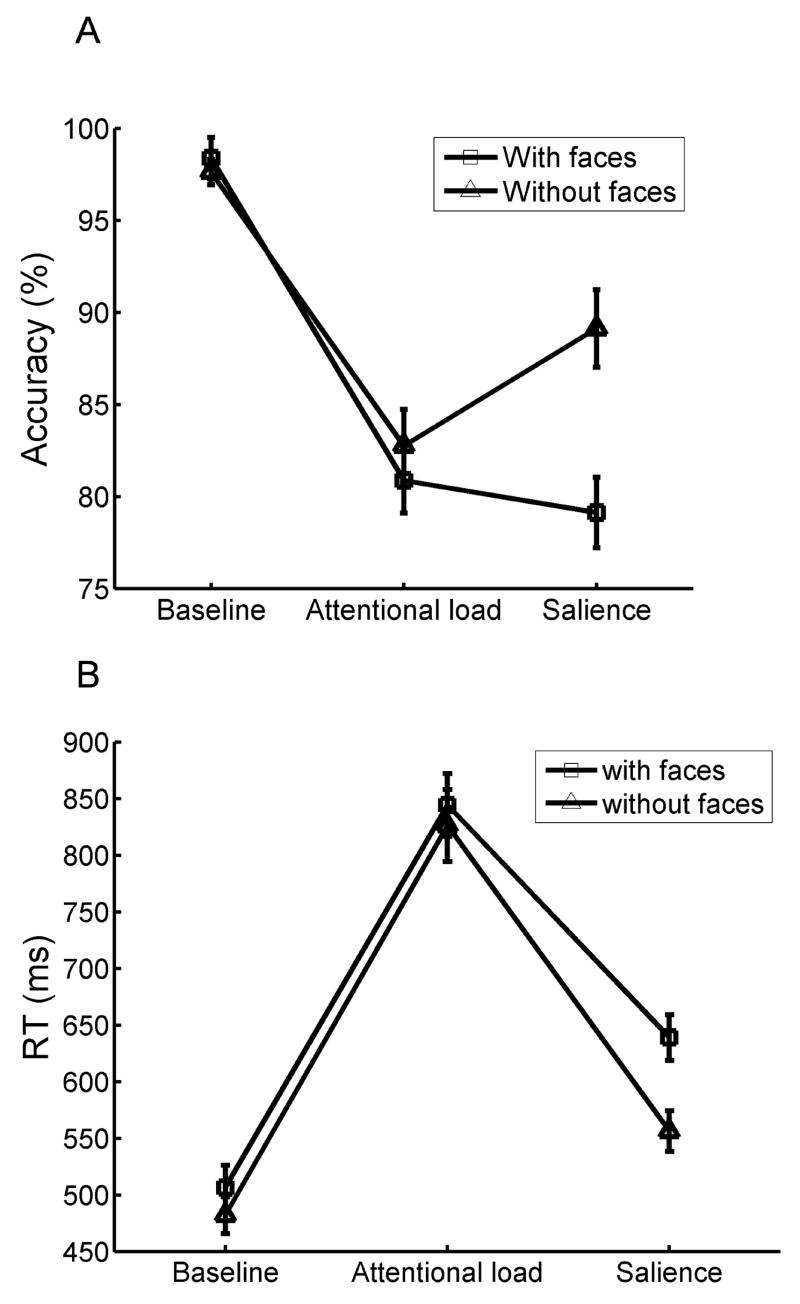
Mean (A) accuracy and (B) reaction time as a function of task manipulations and the presence of face distractors during early trials.
For the early trials, a two-way repeated-measures ANOVA [3 tasks (baseline, attentional load, salience) x 2 distractor types (face, noface)] was conducted on mean reaction time (RT) and accuracy (fearful- and neutral-face trials were averaged for face distractors; only correct trials were included in the RT analysis; the arcsine transformation was employed on the accuracy data to equalize variance). A significant main effect of task was observed for both RT [F (2,38)=165.7, p<0.001] and accuracy [F(2,38)=62.9, p<0.001]. Bonferroni-corrected t tests revealed that RTs were significantly longer (p<0.001) during the attentional load (M=835.3 ms) than during the salience condition (M=597.6 ms), which, in turn, produced longer RTs (p<0.001) than the baseline condition (M=494.3 ms). In terms of accuracy, both the attentional load (M=81.8% correct) and the salience conditions (M=84.1% correct) were significantly harder (p<0.001 in both cases) than the baseline condition (M=98.0% correct). As expected, there were no significant differences in accuracy between the attentional load and salience conditions, given that the parameters during the salience condition were updated to reflect the task difficulty during the attentional load condition. Taken together, the attentional load and salience manipulations produced longer RTs and reduced accuracy relative to the baseline condition.
The two-way ANOVA also revealed a main effect of distractor type both in terms of RTs [F(1,19)=34.7, p<0.001] and accuracy [F(1,19)=6.1, p<0.05]. Mean RTs were longer when face distractors were presented (M=663.3 ms) than when they were absent (M=621.5 ms). In addition, accuracy decreased during conditions with face distractors (M=86.1% correct) compared to conditions without face distractors (M=89.8% correct). These results indicate that face distractors impaired target performance, revealing the presence of distractor interference effects.
Task by distractor interactions during early trials
Significant interactions between task and distractor type were found for RT [F(2,38)=7.7, p<0.01] and accuracy [F(2,38)=17.8, p<0.001]. To further assess how distractor interference effects were modulated by task conditions, Bonferroni-corrected t tests were conducted on RT. When comparing face-present vs. face-absent conditions, participants responded significantly more slowly to targets in both the salience (p<0.001) and the baseline conditions (p<0.01), but no significant difference was observed during the attentional load condition (p=0.9); note the relatively large error bars for the attentional load condition and the relatively small error bars for the baseline condition. In addition, to determine the relative magnitude of distractor effects, RT differences between face-present and face-absent conditions were calculated and submitted to a t test. Larger distractor interference effects were obtained during the salience condition relative to the baseline condition [t(19)=4.4, p<0.001]. In terms of accuracy, Bonferroni-corrected t tests revealed a significant distractor interference effect during the salience condition only (p<0.001). Overall, the data revealed that distractors interfered less with target performance during the attentional load condition. By contrast, the salience manipulation actually increased distractor interference.
Valence effects during early trials
To probe whether fearful and neutral faces had differential effects on target performance, mean RTs and accuracy were submitted to a 3 (task: baseline, attentional load, salience) by 2 (facial expression: fearful, neutral) repeated-measures ANOVA. No significant main effects of facial expressions or interactions were found during either early or late trials (all p’s > 0.1).
Behavioral performance during late trials
The pattern of results during late trials closely followed the one observed during early trials, except that there was no task by distractor (present/absent) interaction on RT. Follow up t tests revealed that when comparing face-present vs. face-absent conditions, participants responded significantly more slowly to targets not only during the baseline (p<0.05) and the salience (p<0.01) conditions but also during the attentional load condition (p<0.01). The presence of an interference effect during the attentional load condition for the late but not for the early trials suggests that participants became better at the search task during the second half of the experiment.
Discussion
In the present study, we investigated how both bottom-up (stimulus salience) and top-down (attentional load) factors affect the processing of task-irrelevant face distractors, as indexed by responses evoked in the amygdala and the fusiform gyrus. Indeed, bottom-up and top-down manipulations exhibited dissociable effects. When the attentional load of the main task was increased relative to baseline, responses evoked by fearful- and neutral-face distractors decreased during the early trials. By contrast, decreasing target salience (possibly increasing the salience of task-irrelevant face distractors) resulted in increased responses relative to the baseline condition during the early trials. Interestingly, time-related effects also depended on the task. During the salience condition, amygdala and fusiform gyrus responses to distractors decreased over the experimental time course. However, surprisingly, during the attentional load condition, responses increased from early to late trials. Taken together, our findings revealed that the processing of task-irrelevant face stimuli can be modulated by both stimulus salience and attentional load, but that such bottom-up and top-down factors have dissociable effects on distractor processing when the task difficulty of the two conditions is equated. Finally, parallel results were observed for fearful- and neutral-face distractors.
Effectiveness of task manipulations
We manipulated the load of the main task by requiring subjects to perform a challenging (attentional load) or a “pop-out” (baseline) search task. During the baseline condition, RTs were slower when face distractors were present relative to non-face conditions, revealing that face distractors interfered with the task (unless otherwise noted, behavioral effects for early trials are reported). During the attentional load condition, however, no significant differences in RT were observed during the face and no-face conditions, consistent with the idea that reduced processing resources were available to process the task-irrelevant faces during the attentional load condition (Lavie, 1995; Lavie & De Fockert, 2003). Finally, during the salience condition, RTs were slower during face vs. no-face trials, again indicating that face distractors interfered with the task. Critically, the interference during the salience condition was greater than during the baseline condition. Because increased distractor salience impairs task performance (Eltiti et al., 2005; Mounts, 2005; Theeuwes, 2005; Yantis & Jonides, 1990), these findings corroborate the notion that our manipulation, indeed, increased the relative salience of the distractors. Overall, our behavioral findings closely agree with a previous behavioral study by Lavie and De Fockert (2003), who reported contrasting effects of sensory and capacity limits during attention tasks.
The impact of the different conditions can also be gauged via the imaging data during the no-face conditions. For instance, the effectiveness of the attentional load manipulation was further evidenced by results showing that, relative to the baseline condition, higher activation was observed in a network of fronto-parietal brain regions that have been linked to important attentional functions (Corbetta & Shulman, 2002; Kastner & Ungerleider, 2000; Pessoa & Ungerleider, 2005). At the same time, the contrast of salience vs. baseline only revealed differential activation in the left SPL, suggesting that the salience condition was not linked to strong capacity-related attentional demands. In the present context, it is also informative that increased responses were evoked in the left fusiform gyrus during the attentional load condition relative to the baseline condition. In line with the involvement of the left fusiform gyrus in letter recognition (Polk et al., 2002), the increased activation may have reflected enhanced processing of the letter array when additional processing resources were allocated to it (Kastner, Pinsk, De Weerd, Desimone, & Ungerleider, 1999).
Isolating the effects of face distractors
A central goal of the present study was to probe the effect of fearful- and neutral-face distractors on the responses evoked in the amygdala and fusiform gyrus. During our tasks, both task-relevant (letter array) and task-irrelevant (faces) stimuli were present in the display. In addition, in all cases, subjects performed a detection task in which they had to detect a target letter among uniform or non-uniform nontarget letters. Thus, evoked responses may have reflected contributions from the face stimuli, the letter array, in addition to the task itself. For instance, previous studies have shown that amygdala activity is modulated by non-emotional tasks (Drevets & Raichle, 1998), and we have shown that performing neutral, difficult attentional tasks decreases amygdala responses relative to fixation (Pessoa et al., 2005). To factor out the effects of the letter array and the task, in the present study, we employed three conditions that contained only the letter arrays (no-face conditions) and that involved the same tasks that subjects performed when face distractors were present. In this fashion, our design allowed us to isolate the contributions of face distractors to evoked responses. Note that by subtracting out the no-face conditions, we also minimized any potential effects of stimulus differences in the displays across the experimental conditions.
Dissociable task effects: Capacity vs. sensory demands
The attentional load condition was designed to consume resources that would be available to process distractor faces. In designing the salience condition, we sought to create a second condition that was equivalent in terms of task difficulty, but which did not impose the same type of capacity demands. Instead, we employed a task that was more difficult because of sensory degradation (Lavie & De Fockert, 2003). Our results revealed that, in fact, the attentional load and the salience conditions had dissociable effects on distractor processing.
The comparison of RTs during the salience condition with and without faces revealed that the distractors interfered with the main task by slowing down performance. Such interference effect was reduced during the attentional load condition, where differences in RT with and without faces did not reach significance. The neuroimaging data also provided information about the extent of distractor processing and interference effects. During the early trials of the experiment, evoked responses in the amygdala and the fusiform gyrus to both fearful and neutral faces were weaker during the attentional load condition relative to the baseline. The behavioral and fMRI data thus suggest that increasing the resource demands of the main task decreased distractor processing, which, in turn, decreased distractor interference. By contrast, stronger amygdala and fusiform gyrus activity was observed during the salience condition relative to the baseline. In this case, the behavioral and fMRI data suggest enhanced distractor processing, which was associated with increased distractor interference. Overall, on the one hand, the results of the attentional load condition are consistent with previous research in which the extent of distractor processing depends on available processing resources (Lavie, 1995, 2005). On the other hand, the results of the salience condition are compatible with the view that stimulus salience also plays an important role in distractor processing (Eltiti et al., 2005; Mounts, 2005; Theeuwes, 2005; Yantis & Jonides, 1990). In addition, our findings revealed that responses to unattended neutral and fearful faces depend on the type of task manipulation, and not task difficulty per se. Our work thus distinguishes the role of bottom-up factors (salience) from top-down factors (attentional load) in determining the fate of task-irrelevant stimuli.
Although the attentional load and salience conditions were equated in terms of accuracy, responses were significantly faster during the latter condition. It is thus possible that some of the differential effects that we observed were due to differences in RT. Note, however, that RTs during the salience condition were faster than during the attentional load condition, suggesting that increased distractor processing during the salience condition was not due to simple time-on-task effects. In addition, although the baseline condition produced the shortest RTs, distractor-related responses were not stronger than during the other conditions. Thus, we suggest that the dissociation in distractor processing during the attentional load and salience conditions reflected the type of demand imposed by the main task, as suggested above.
As stated above, we observed dissociable effects of the attentional load and the salience conditions on distractor processing. Nevertheless, it should be noted that we do not view our bottom-up and top-down manipulations as mutually exclusive. For instance, when comparing the baseline and salience conditions, the same type of singleton search was involved, whereas the size and contrast of the letters was reduced in the salience condition. Although we employed this manipulation to probe the role of bottom-up factors, it is possible that this manipulation also affected top-down factors – for instance, the spatial focus of attention may have been narrowed during the salience condition relative to baseline. The contrasting effects observed during the attentional load and salience conditions argue, however, that, if the salience condition also affected top-down factors, that such factors did not completely overlap with those manipulated during the attentional load condition.
Differential time-related responses
A persisting response to an unchanging stimulus is metabolically expensive and conveys little information. Thus, it would be beneficial to attenuate evoked responses to a persisting stimulus. Indeed, previous findings have shown that amygdala and fusiform gyrus activity rapidly attenuate to repetitions of face stimuli (Breiter et al., 1996; Grill-Spector et al., 1999). In our experiment, response attenuation to face distractors was found during the salience condition. We suggest that such attenuation was directly linked to the robust responses during this condition in both the amygdala and the fusiform gyrus, such that response attenuation was, in fact, a consequence of the increased distractor processing during the salience condition. This interpretation is consistent with a previous study that reported a greater degree of suppression to repetitions of fearful faces, which evoked the strongest responses, relative to the degree of suppression of neutral faces, which evoked weaker responses (Ishai et al., 2004). This line of reasoning would predict that reduced attenuation should be observed when responses are less vigorous, as during the attentional load condition. Our results are partly consistent with this interpretation, as we observed response potentiation when comparing early and late trials of the attentional load condition. Given the presence of an interference effect during late but not during the early trials, one potential explanation for such effect is that, with time, participants became better at the letter-detection task, “releasing” some processing resources, which could then be applied more effectively towards the processing of the face distractors. Nevertheless, additional studies are needed to further clarify the response potentiation that occurred during the attentional load condition.
Valence effects
The role of the amygdala in the processing of fear has been repeatedly demonstrated in both neuropsychological and neuroimaging studies (Adolphs et al., 1994; Breiter et al., 1996; Young et al., 1995). In line with this notion, the present experiment showed that the amygdala exhibited greater evoked responses to fearful distractors relative to neutral distractors during all task conditions, during the early trials. However, during the late trials, valence effects persisted during the attentional load condition. We suggest that such pattern of results parallels the time-related data reported earlier. Because of the attenuated processing of distractors during the attentional load condition, the valence effect was still observed during the late trials. It is important to note, however, that because fearful and neutral faces differed both in terms of valence and arousal, the present study cannot separate their specific contributions. Accordingly, in the present context, “valence” effects should be viewed more generally as valence/arousal effects.
Effects of task manipulations on face processing
Some studies have supported the view that the perception of fearful faces is independent of attention, such that they would not be subject to limited processing capacity constraints (Anderson et al., 2003; Vuilleumier et al., 2001; Williams et al., 2005). In the present study, we found that the perception of both neutral and fearful faces was modulated by a top-down factor (attentional load). In addition, salience also affected the processing of neutral and fearful faces. Critically, no task by valence interaction was observed, suggesting that, in the present experiment, both neutral and fearful faces were affected by our task manipulations in a similar fashion. Although most of the previous studies supporting attention-independent processing employed moderately challenging “main” tasks, task difficulty may have different effects on distractor processing as shown in the present experiment, as well as previous studies (De Fockert, Rees, Frith, & Lavie, 2001; Lavie & De Fockert, 2003; Lavie, Hirst, De Fockert, & Viding, 2004). Thus, the extent to which tasks adopted in previous studies effectively consumed processing resources is unclear. In addition, in previous studies, we showed that differential responses to fearful vs. neutral faces were abolished during very demanding conditions (Pessoa et al., 2002; Pessoa et al., 2005). A second factor that should be considered when interpreting previous studies refers to potential habituation and other related time-dependent effects. As revealed in the present study, different conditions were associated with varying amounts of habituation and/or potentiation of evoked responses.
Finally, it has been suggested that different types of non-emotional tasks modulate amygdala responses, especially in a suppressive manner (Drevets & Raichle, 1998). In the present study, responses evoked in the amygdala during both the salience and attentional load conditions when no face distractors were present were weaker than during the baseline condition, suggesting that both of these relatively difficult task conditions “suppressed” amygdala activation. Thus, it is important to account for such effects, for instance, by including conditions in which only the stimuli of the main task are displayed. Otherwise, activation in the amygdala and other regions may be at least partly confounded by the effects of the main tasks.
In keeping with previous findings, the current research underscores the privileged status of fear processing in the human brain – as evidenced by stronger responses evoked by fearful relative to neutral faces. In addition, our experiment revealed that the processing of unattended fearful faces can be shaped by both bottom-up and top-down factors, just like the processing of unattended neutral faces. Overall, our findings add to our understanding of the processing of emotion-laden information in the brain and suggest that responses evoked by this class of stimuli depend on a wide array of factors that ultimately determine the strength of evoked responses.
Acknowledgments
We thank the National Institute of Mental Health for support for this work (award 1R01 MH071589 to L.P.), and the Ittleson Foundation. We also would like to thank Eswar Damaraju and Srikanth Padmala for technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired Recognition Of Emotion In Facial Expressions Following Bilateral Damage To The Human Amygdala. Nature. 1994;372(6507):669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Panitz D, De Rosa E, Gabrieli JDE. Neural correlates of the automatic processing of threat facial signals. Journal of Neuroscience. 2003;23(13):5627–5633. doi: 10.1523/JNEUROSCI.23-13-05627.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17(5):875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Broadbent DE. Perception and communication. London: Pergamon; 1958. [Google Scholar]
- Bundesen C. A theory of visual-attention. Psychological Review. 1990;97(4):523–547. doi: 10.1037/0033-295x.97.4.523. [DOI] [PubMed] [Google Scholar]
- Compton RJ. The interface between emotion and attention: A review of evidence from psychology and neuroscience. Behavioral and cognitive neuroscience reviews. 2003;2:115–129. doi: 10.1177/1534582303255278. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- De Fockert JW, Rees G, Frith CD, Lavie N. The role of working memory in visual selective attention. Science. 2001;291(5509):1803–1806. doi: 10.1126/science.1056496. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual-attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Deutsch JA, Deutsch D. Attention: Some theoretical considerations. Psychological Review. 1963;70(1):80–90. doi: 10.1037/h0039515. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implications for interactions between emotion and cognition. Cognition & Emotion. 1998;12(3):353–385. [Google Scholar]
- Ekman P, Friesen WV. Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- Eltiti S, Wallace D, Fox E. Selective target processing: Perceptual load or distractor salience? Perception & Psychophysics. 2005;67(5):876–885. doi: 10.3758/bf03193540. [DOI] [PubMed] [Google Scholar]
- Erthal FS, de Oliveira L, Mocaiber I, Pereira MG, Machado-Pinheiro W, Volchan E, et al. Load-dependent modulation of affective picture processing. Cognitive Affective & Behavioral Neuroscience. 2005;5(4):388–395. doi: 10.3758/cabn.5.4.388. [DOI] [PubMed] [Google Scholar]
- Fecteau JH, Munoz DP. Salience, relevance, and firing: a priority map for target selection. Trends in Cognitive Sciences. 2006;10(8):382–389. doi: 10.1016/j.tics.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Fischer H, Wright CI, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Brain habituation during repeated exposure to fearful and neutral faces: A functional MRI study. Brain Research Bulletin. 2003;59(5):387–392. doi: 10.1016/s0361-9230(02)00940-1. [DOI] [PubMed] [Google Scholar]
- Globisch J, Hamm AO, Esteves F, Öhman A. Fear appears fast: Temporal course of startle reflex potentiation in animal fearful subjects. Psychophysiology. 1999;36(1):66–75. doi: 10.1017/s0048577299970634. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Edelman S, Avidan G, Itzchak Y, Malach R. Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron. 1999;24(1):187–203. doi: 10.1016/s0896-6273(00)80832-6. [DOI] [PubMed] [Google Scholar]
- Grossberg S. How does a brain build a cognitive code. Psychological Review. 1980;87 (1):1–51. doi: 10.1007/978-94-009-7758-7_1. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4(6):223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Shallice T, Gorno-Tempini ML, Dolan RJ. Face repetition effects in implicit and explicit memory tests as measured by fMRI. Cerebral Cortex. 2002;12(2):178–186. doi: 10.1093/cercor/12.2.178. [DOI] [PubMed] [Google Scholar]
- Holmes A, Vuilleumier P, Eimer M. The processing of emotional facial expression is gated by spatial attention: evidence from event-related brain potentials. Cognitive Brain Research. 2003;16(2):174–184. doi: 10.1016/s0926-6410(02)00268-9. [DOI] [PubMed] [Google Scholar]
- Holmes A, Winston JS, Eimer M. The role of spatial frequency information for ERP components sensitive to faces and emotional facial expression. Cognitive Brain Research. 2005;25(2):508–520. doi: 10.1016/j.cogbrainres.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Ishai A, Pessoa L, Bikle PC, Ungerleider LG. Repetition suppression of faces is modulated by emotion. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(26):9827–9832. doi: 10.1073/pnas.0403559101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22(4):751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Lavie N. Perceptual load as a necessary condition for selective attention. Journal of Experimental Psychology-Human Perception And Performance. 1995;21(3):451–468. doi: 10.1037//0096-1523.21.3.451. [DOI] [PubMed] [Google Scholar]
- Lavie N. Distracted and confused?: Selective attention under load. Trends in Cognitive Sciences. 2005;9(2):75–82. doi: 10.1016/j.tics.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Lavie N, De Fockert JW. Contrasting effects of sensory limits and capacity limits in visual selective attention. Perception & Psychophysics. 2003;65(2):202–212. doi: 10.3758/bf03194795. [DOI] [PubMed] [Google Scholar]
- Lavie N, Hirst A, De Fockert JW, Viding E. Load theory of selective attention and cognitive control. Journal of Experimental Psychology-General. 2004;133(3):339–354. doi: 10.1037/0096-3445.133.3.339. [DOI] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Öhmann A. The Karolinska directed emotional faces (KDEF) Stockholm: Karolinska Institute; 1998. [Google Scholar]
- Mounts JRW. Attentional selection: A salience-based competition for representation. Perception & Psychophysics. 2005;67(7):1190–1198. doi: 10.3758/bf03193552. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Joseph JS. Attention, pattern recognition and popout in visual search. In: Parasuraman R, editor. The Attentive Brain. Cambridge: MIT Press; 1998. pp. 279–298. [Google Scholar]
- Norman DA, Bobrow D. On data-limited and resource-limited processes. Cognitive Psychology. 1975;7:44–64. [Google Scholar]
- Öhman A, Esteves F, Soares JJF. Preparedness and preattentive associative learning: Electrodermal conditioning to masked stimuli. Journal of Psychophysiology. 1995;9(2):99–108. [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(17):11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Padmala S, Morland T. Fate of unattended fearful faces in the amygdala is determined by both attentional resources and cognitive modulation. Neuroimage. 2005;28(1):249–255. doi: 10.1016/j.neuroimage.2005.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Ungerleider LG. Top-down mechanisms for working memory and attentional processes. In: Gazzaniga MS, editor. The cognitive neurosciences. 3. Cambridge, Massachusetts: The MIT press; 2005. pp. 919–930. [Google Scholar]
- Phillips ML, Medford N, Young AW, Williams L, Williams SCR, Bullmore ET, et al. Time courses of left and right amygdalar responses to fearful facial expressions. Human Brain Mapping. 2001;12(4):193–202. doi: 10.1002/1097-0193(200104)12:4<193::AID-HBM1015>3.0.CO;2-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polk TA, Stallcup M, Aguirre GK, Alsop DC, D'Esposito M, Detre JA, et al. Neural specialization for letter recognition. Journal of Cognitive Neuroscience. 2002;14(2):145–159. doi: 10.1162/089892902317236803. [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, Asgari M, Gore JC, McCarthy G. Differential sensitivity of human visual cortex to faces, letterstrings, and textures: A functional magnetic resonance imaging study. Journal of Neuroscience. 1996;16(16):5205–5215. doi: 10.1523/JNEUROSCI.16-16-05205.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theeuwes J. Irrelevant singletons capture attention. In: Itti L, Rees G, Tsotsos J, editors. Neurobiology of Attention. San Diego, CA: Elsevier; 2005. [Google Scholar]
- Treisman A, Gormican S. Feature analysis in early vision: Evidence from search asymmetries. Psychological Review. 1988;95(1):15–48. doi: 10.1037/0033-295x.95.1.15. [DOI] [PubMed] [Google Scholar]
- Treisman AM. Contextual cues in selective listening. Quarterly Journal of Experimental Psychology. 1960;12(4):242–248. [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: An event-related fMRI study. Neuron. 2001;30(3):829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Williams LM, Brown KJ, Das P, Boucsein W, Sokolov EN, Brammer MJ, et al. The dynamics of cortico-amygdala and autonomic activity over the experimental time course of fear perception. Cognitive Brain Research. 2004;21(1):114–123. doi: 10.1016/j.cogbrainres.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Williams MA, McGlone F, Abbott DF, Mattingley JB. Differential amygdala responses to happy and fearful facial expressions depend on selective attention. Neuroimage. 2005;24(2):417–425. doi: 10.1016/j.neuroimage.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. Visual search. In: Pashler H, editor. Attention. London: University College London Press; 1998. pp. 13–74. [Google Scholar]
- Wright CI, Fischer H, Whalen PJ, McInerney S, Shin LM, Rauch SL. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport. 2001;12(2):379–383. doi: 10.1097/00001756-200102120-00039. [DOI] [PubMed] [Google Scholar]
- Yantis S, Jonides J. Abrupt visual onsets and selective attention: Voluntary versus automatic allocation. Journal of Experimental Psychology-Human Perception and Performance. 1990;16(1):121–134. doi: 10.1037//0096-1523.16.1.121. [DOI] [PubMed] [Google Scholar]
- Young AW, Aggleton JP, Hellawell DJ, Johnson M, Broks P, Hanley JR. Face processing impairments after amygdalotomy. Brain. 1995;118:15–24. doi: 10.1093/brain/118.1.15. [DOI] [PubMed] [Google Scholar]