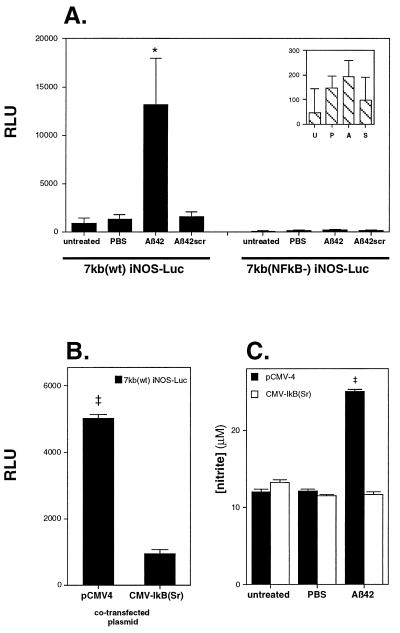
Figure 5.
Aβ42 stimulates iNOS in astrocytes in an NFκB-dependent manner. (A) Aβ42-stimulated iNOS promoter activity as determined by luciferase activity. Astrocytes were transfected with the 7-kb iNOS promoter luciferase reporter construct [7kb(wt) iNOS-LUC] and then either left untreated or stimulated by PBS, 10 μM Aβ42, or 10 μM Aβ42scr for 12 hr. Only Aβ42 significantly stimulated iNOS promoter activity. Inactivation of the TATAA-box proximal NFκB response element in the 7-kb iNOS promoter by site-directed mutagenesis [7kb(NFκB−) iNOS-LUC] results in no significant promoter activity by Aβ42. (Inset) The same 7kb(NFκB−) iNOS-LUC RLU data with a more focused ordinate range. U, P, A, and S are untreated, PBS-, Aβ42-, and Aβ42scr-treated cells, respectively. Data (mean RLU ± SEM) represent n = 8 transfections. (B) Cotransfecting the 7-kb iNOS-LUC construct with CMV-IκB(Sr) reduced Aβ-stimulated iNOS promoter activity to near background levels compared with cotransfection of 7kb(wt) iNOS-LUC with the backbone vector pCMV4. Data (mean RLU ± SEM) represent n = 6 transfections. (C) iNOS activity was determined by measuring the production of nitrite by a modified Griess assay as described. Aβ42-stimulated nitrite production was reduced to background control levels in astrocytes transfected with CMV-IκB(Sr), but not in Aβ42-stimulated astrocytes transfected with backbone vector pCMV4 alone. (Each transfection well received a total of 750 ng of plasmid DNA. Control backbone vector transfection wells received 750 ng of pCMV4 and CMV-IκB(Sr) transfection wells received 0.75 ng of CMV-IκB(Sr) plus 749.25 ng of pCMV4.) Data (mean ± SEM) represent n = 11 transfections. ∗, Significantly different from PBS control (P < 0.05); ‡, significantly different from CMV-IκB(Sr) (P < 0.005).