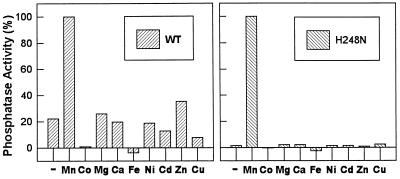
Figure 1.
Metal ion dependence of wild-type PP-1 and the H248N mutant. Metals were removed by Mono-Q chromatography before assay. Each enzyme was diluted in 50 mM Tris·HCl buffer (pH 7.0), containing 10% glycerol, 0.1% 2-mercaptoethanol, and 2 mM EDTA. The enzyme was eluted with a gradient of 0.1–0.5 M NaCl in the equilibration buffer, and the fractions containing the most Mn2+-dependent enzyme activity were pooled. Enzyme activity was measured in the absence (−) or presence (+) of 1 mM metal chloride as indicated. (Left) Wild type; (Right) H248N. Enzyme activities were normalized (see Table 1 for values).