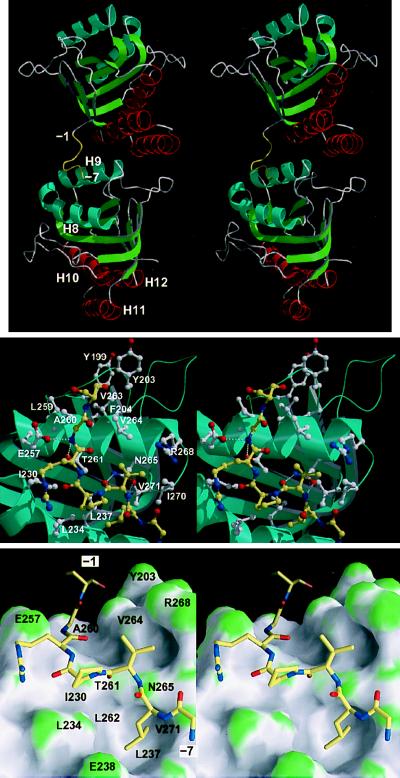
Figure 2.
(Top) Stereo cartoon representation of the structure of the mini-chaperone (GroEL191–376), showing the interaction between the N-terminal tag and a neighboring molecule in the crystal lattice (related by a crystallographic two-fold screw operation along the c axis, positioned approximately vertical and in the plane of the paper). The N-terminal tag (residues −1 to −7) is colored yellow. (Middle) Close-up of peptide-binding site interactions, in stereo. The peptide is represented by yellow bonds; neighboring residues are represented by white bonds. Hydrogen bonds are represented by broken white lines. Drawn with bobscript (extensions to the program molscript; ref. 24) and raster3d (25). (Lower) As in Middle but showing the molecular surface of the mini-chaperone. The surface is colored according to surface curvature to highlight concave surface pockets. Convex, concave, and flat surfaces are colored green, grey, and white, respectively. Residues underlying the surface are labeled. Drawn with grasp (26). All three figures show the model in approximately the same orientation.