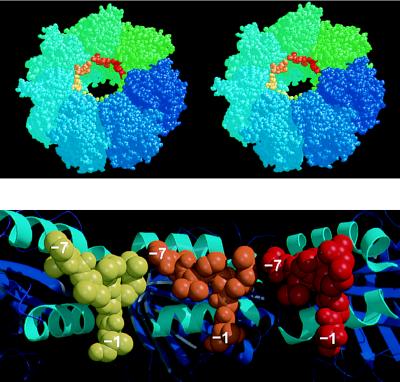
Figure 4.
(Upper) Stereoview of one heptameric ring of the GroEL tetradecamer showing the position of the N-terminal tag bound to each apical domain, near the opening to the central cavity. This model is generated by the superposition of the mini-chaperone GroEL(191–376) with each corresponding apical domain (residues 191–376) in intact GroEL (the second ring of the GroEL cylinder, generated by a two-fold symmetry operation, is not shown, but stacks against the underside of the drawn ring). GroEL subunits are colored around the ring going from blue to green. Superimposed “bound peptides” are colored from green to red. Drawn with rasmol (32). (Lower) Cross-section of the model shown in Upper looking directly at the inner wall of the cavity, and showing the apical domains (cartoon with helices H8 and H9 colored cyan) from three subunits with modeled peptide (shown as space-filling models colored yellow, orange, and red, respectively). Drawn with molscript (24) and raster3d (25). Each of the separate peptides (residues −1 to −7) could be linked together by small fragments of peptides so that a longer peptide could bind from one contiguous site to the next.