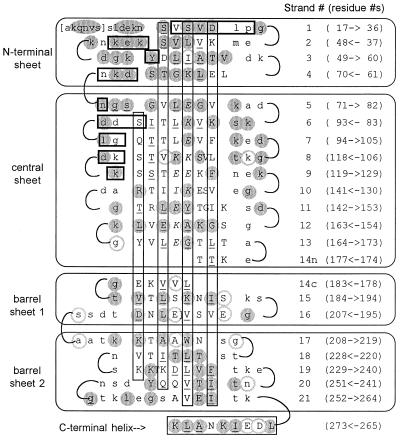
Figure 2.
Two-dimensional representation of the OspA folding pattern. The view is of the front face of the central β-sheet, with other β-sheets “uncurled” to form a single pseudocontinuous sheet. Residues in secondary structural elements (β-strands or α-helix) are represented by uppercase type; residues in turns are in lowercase type. Residues in brackets at the N terminus are predicted to be present in recombinant OspA but are not seen in the crystal. The four β-sheets formed by the 21 antiparallel β-strands are indicated by labeled rectangles enclosing sections of sequence, and the C-terminal α-helix is indicated at the bottom. For each line, the β-strand number is indicated in parentheses to the right, followed by the numerical range and direction of displayed residues. Vertical rectangles indicate residues with side chains extending behind the plane of the figure, residues with uppercase type outside the rectangles extend toward the viewer. Residues with exposed surface areas less than 10 Å2 are considered buried and are underlined. In general, buried residues are front-facing between strands 1 and 9 and rear-facing between strands 11 to 21, corresponding to the folding of N- and C-terminal domains on opposite sides of the central sheet. Strand 14 has main-chain hydrogen bonds to both the central sheet (14n) and to barrel sheet 1 (14c). Residues that contact Fab 184.1 are horizontally boxed (heavy lines); residues in the charge arrays are indicated in italic type. Residues strictly conserved in 49 OspA sequences (see Fig. 4) are within shaded circles; highly variable residues are within circles with shaded outlines.